
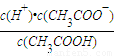 ��0.10mol/L�Ĵ�����Һ��pHԼΪ______����ʾ������ĵ��볣����С��ƽ��ʱ��c��CH3COOH���ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3=0.114����
��0.10mol/L�Ĵ�����Һ��pHԼΪ______����ʾ������ĵ��볣����С��ƽ��ʱ��c��CH3COOH���ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3=0.114����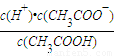 ��0.10mol/L�Ĵ�����Һ��c��H+��=
��0.10mol/L�Ĵ�����Һ��c��H+��=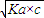 ���Դ˼���pH��
���Դ˼���pH��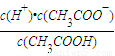 ��0.10mol/L�Ĵ�����Һ��c��H+��=
��0.10mol/L�Ĵ�����Һ��c��H+��=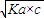 =
=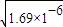 =1.3×10-3mol/L����pH=2.88���ʴ�Ϊ��2.88��
=1.3×10-3mol/L����pH=2.88���ʴ�Ϊ��2.88��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| c(H+)?c(CH3COO-) | c(CH3COOH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�(��֪��25�棬Ka(CH3COOH)��1.69��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pHԼΪ ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)
�ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3��0.114����
�� ���ڴ�����Һ�ʹ�������Һ������˵����ȷ����
A��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С��
�����¶ȿ��Դٽ�������룬�������¶�������ƴ�����ˮ�⡣
C������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ��롣
D������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ��롣
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������ϣ�ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��c(CH3COOH)��2c(H��)��c(CH3COO��)��2c(OH��)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
�� ����ʱ��������3����Һ������pH��С����

�� ��֪������![]() ����С�մ���Һ�������з�Ӧ��
����С�մ���Һ�������з�Ӧ��
CH3COOH��NaHCO3��CH3COONa��CO2����H2O ��
��pH��ֽ�ڳ����·ֱ�ⶨ![]() 0.10mol/L�Ĵ�������Һ��0.10mol/L��̼��������Һ��
0.10mol/L�Ĵ�������Һ��0.10mol/L��̼��������Һ��
��pH(CH3COONa) pH(NaHCO3)�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�(��֪��25�棬Ka(CH3COOH)��1.69��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���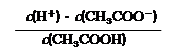
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pHԼΪ ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)
�ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3��0.114����
�� ���ڴ�����Һ�ʹ�������Һ������˵����ȷ����
A��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С��
�����¶ȿ��Դٽ�������룬�������¶�������ƴ�����ˮ�⡣
C������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ��롣
D������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ��롣
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������ϣ�ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��c(CH3COOH)��2c(H��)��c(CH3COO��)��2c(OH��)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
�� ����ʱ��������3����Һ������pH��С����
�� ��֪�����ܹ���С�մ���Һ�������з�Ӧ��
CH3COOH��NaHCO3��CH3COONa��CO2����H2O��
��pH��ֽ�ڳ����·ֱ�ⶨ0.10mol/L�Ĵ�������Һ��0.10mol/L��̼��������Һ��
��pH(CH3COONa) pH(NaHCO3)�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�
(��֪��25�棬����ĵ���ƽ�ⳣ��Ka(CH3COOH)��1��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pH���� ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)�ɽ�����Ϊ�Ե���0.10mol/L������
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������Ϻ���Һ��PH<7��ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��2c(Na��) ��c(CH3COO��)��c(CH3COOH)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
D��c(Na��)>c(CH3COO��)> c(H��)> c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�ܿ��и߶���ѧ�����п��Ի�ѧ�� ���ͣ������
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�(��֪��25�棬Ka(CH3COOH)��1.69��10��5���� ��ش�
�� д����������ˮ�з���ˮ�ⷴӦ�����ӷ���ʽ�� ��
�� ��CH3COONa��Һ������Ũ���ɴ�С��˳��Ϊ
���á�c(Bn��)����ʾ��Ӧ����Ũ�ȣ���
�� 25��ʱ������ĵ���ƽ�ⳣ������ʽKa�� ��0.10mol/L�Ĵ�����Һ��pHԼΪ ����ʾ������ĵ��볣����С��ƽ��ʱ��c(CH3COOH)
�ɽ�����Ϊ�Ե���0.10mol/L�� ��֪��lg1.3��0.114����
�� ���ڴ�����Һ�ʹ�������Һ������˵����ȷ����
A��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С��
�����¶ȿ��Դٽ�������룬�������¶�������ƴ�����ˮ�⡣
C������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ��롣
D������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ��롣
�� ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONa��CH3COOH��Һ�������ϣ�ע�����ǰ����Һ����仯���Բ��ƣ������Һ�е����й�ϵʽ��ȷ���� ��
A��c(CH3COOH)��2c(H��)��c(CH3COO��)��2c(OH��)
B��c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
C��c(CH3COO��)��c(CH3COOH)��0.1mol/L
�� ����ʱ��������3����Һ������pH��С����

�� ��֪�����ܹ���С�մ���Һ�������з�Ӧ��
CH3COOH��NaHCO3��CH3COONa��CO2����H2O ��
��pH��ֽ�ڳ����·ֱ�ⶨ0.10mol/L�Ĵ�������Һ��0.10mol/L��̼��������Һ��
��pH(CH3COONa) pH(NaHCO3)�������������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com