
| A���ζ�ʱ���۾�Ӧʼ��ע�ӵζ�����Һ��ı仯 |
| B���ü�ʽ�ζ�����ȡ0.10 mol��L��1��KMnO4��Һ15.10 mL |
| C������к͵ζ�֮ǰ����ƿ������ˮϴ�����ɣ������ô���Һ��ϴ |
| D����pH��ֽ����ij��Һ��pHʱҪ�Ƚ���ֽ��ʪ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��� | A | B |
| �� | �õιܴ��Լ�ƿ����ȡҺ�� | |
| �� | ������������������Ƥ������Щ | |
| �� | Ϊʹ���ȵ����������Һ�岻���� | |
| �� | �����Թ���������״п | |
| �� | ʵ���ұ���Һ�壬����Һ��ӷ��� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
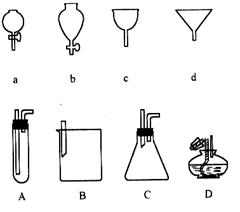
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 mL��Ͳ����100mL����ƿ����250mL��Ͳ������ƽ����50
mL��Ͳ����100mL����ƿ����250mL��Ͳ������ƽ����50 mL�ձ���Ӧѡ�õ������У����ţ� ��
mL�ձ���Ӧѡ�õ������У����ţ� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����CCl4 | B�������屽 | C������ˮ | D���ƾ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������Ʊ�����ú���� |
| B���������Ʊ���������ƿ�� |
| C����״̼��ƹ��屣����ϸ�ڲ���ƿ�� |
| D��ϡ����ɱ�����ϸ��ĥɰ����ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������̨ | B���ձ� | C�������� | D���ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B������ʱ��Ϊ�˷�ֹҺ�屩�У���Ҫ��������ƿ�мӼ�Ƭ���Ƭ |
| C������һ�����ʵ���Ũ�ȵ�NaCl��Һʱ�����ƺõ�NaCl����ֱ�ӷŵ�����ƿ�ܽ� |
| D������ʱ����Һ��ȫ���ɣ���ֹͣ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢۢܢ� | B���ۢܢߢ� | C���ۢܢ� | D���٢ۢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com