
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪�ֹ�����ʵ����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��9�֣�������ѧ֪ʶ���������Ҫ��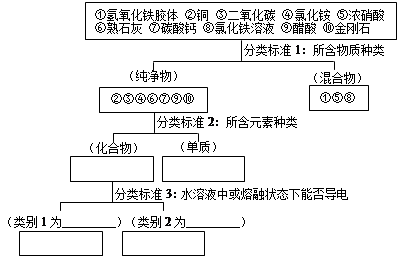
��1��������������� �����1���ܢ���� ��
����2������ ��
��2����ʵ�����Ʊ�������������ķ����ǣ���100 mL�ձ��м���50 mLˮ�����������ڣ� ��
����������������Ȼ�����Һ������Ϻ��ѽ������֣�����˵����ȷ����_________
A���������������н���ֱ������1nm��100nm֮��
B�����ߵı����������������������ж����ЧӦ
C���������������ɢ�����Ӳ�������Ĥ������ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�����и�����ѧ����ĩ���Խ�ѧ������⻯ѧ�Ծ� ���ͣ�ʵ����
��6�֣�
��1������������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò��������� ����д��ţ�
�پ����ᴿ
������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ
�۽������Ȼ���������Һ�����ˮ���Ʊ�������������
��̽��Ba��OH��2��8H2O�����NH4Cl���巴Ӧ�����е������仯
��ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe��OH��2��ɫ����
��2����գ�
�ٶ�ȡ�ζ����е������ʱ������Һ�棬��ȡ������� ʵ����������>������=����<����
����������ƽ��ȡ10.4gʳ�Σ��������ʳ�ε�λ�õߵ�������ȡʳ�ε�����Ϊ g��
��ʵ������480mL 0.1mol��L-1NaOH��Һ��������Һʱ�����ý�ͷ�ιܡ��ձ���������ƽ�������룩����������ҩ���⣬�������õ��������� �������ƣ�������ȡNaOH���������Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪�ֹ�����ʵ����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣�������ѧ֪ʶ���������Ҫ��
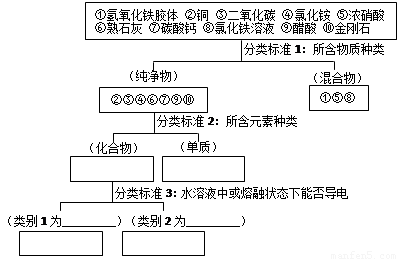
��1��������������� �����1���ܢ���� ��
����2������ ��
��2����ʵ�����Ʊ�������������ķ����ǣ���100 mL�ձ��м���50 mLˮ�����������ڣ� ��
����������������Ȼ�����Һ������Ϻ��ѽ������֣�����˵����ȷ����_________
A���������������н���ֱ������1 nm��100 nm֮��
B�����ߵı����������������������ж����ЧӦ
C���������������ɢ�����Ӳ�������Ĥ������ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и�����ѧ����ĩ���Խ�ѧ������⻯ѧ�Ծ� ���ͣ����
��6�֣�
��1������������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò��������� ����д��ţ�
�پ����ᴿ
������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ
�۽������Ȼ���������Һ�����ˮ���Ʊ�������������
��̽��Ba��OH��2��8H2O�����NH4Cl���巴Ӧ�����е������仯
��ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe��OH��2��ɫ����
��2����գ�
�ٶ�ȡ�ζ����е������ʱ������Һ�棬��ȡ������� ʵ����������>������=����<����
����������ƽ��ȡ10.4gʳ�Σ��������ʳ�ε�λ�õߵ�������ȡʳ�ε�����Ϊ g��
��ʵ������480mL 0.1mol��L-1NaOH��Һ��������Һʱ�����ý�ͷ�ιܡ��ձ���������ƽ�������룩����������ҩ���⣬�������õ��������� �������ƣ�������ȡNaOH���������Ϊ g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com