
��������ʵ��������������ó��Ľ�����ȷ����
���� ���� ����
A ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� �²���Һ����ɫ ԭ��Һ����I��
B ��ij��Һ���ȵμ�KSCN��Һ���ٵμ�������ˮ ��������������Һ���Ѫ��ɫ ԭ��Һ�к���Fe2����Fe3��
C ��ͭƬ����Ũ������ ������������ɫ���壬��Һ��Ϊ����ɫ Ũ������ǿ�����Ժ�����
D ��Na2SiO3��Һ�еμ�1�η�̪��Ȼ����μ���ϡ��������ɫ��ȥ 2 min���Թ���������� ���ԣ��������
AC
��������
���������A���²���Һ����ɫ��˵����Ӧ���е��ʵ����ɡ�������ˮ����ǿ�����ԣ����ԭ��Һ�к��е����ӣ�A��ȷ��B���������ܺ�KSCN��Һ��Ӧʹ��Һ�Ժ�ɫ��������ij��Һ���ȵμ�KSCN��Һ���ٵμ�������ˮ������������������Һ���Ѫ��ɫ����˵��ԭ��Һ�к���Fe2����������Fe3����B����ȷ��C��Ũ�������ǿ�����ԣ��ܰ�ͭ������������ͭ��ˮ��NO2����˲�����������ɫ���壬��Һ��Ϊ����ɫ��C��ȷ��D�����ݽ�ǿ�����Ʊ��������ԭ����֪�����������������������ԣ�D����ȷ����ѡAC��
���㣺�������Ӽ��顢�������ʡ�����ǿ���Ƚ��Լ�ʵ�鷽����������۵�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
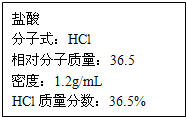 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺| 1000Vd |
| 22400+36.5V |
| 1000Vd |
| 22400+36.5V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
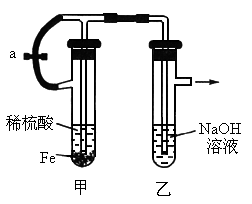
��������ʵ��װ�úͲ������ش��й����⣺
(1)��ͼʾ���Ӻ�װ�ã���ֹˮ��a�������пɹ۲쵽��������________��
(2)��ӦƬ�̺н�ֹˮ��a����ʱ�ɹ۲쵽��������________________�����з�����Ӧ�����ӷ���ʽ��________________________��
(3)���Թ����еĻ���ﵹ��������У��ɹ۲쵽��������________��������Ӧ�ķ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
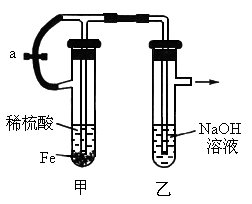
��������ʵ��װ�úͲ������ش��й����⣺
(1)��ͼʾ���Ӻ�װ�ã���ֹˮ��a�������пɹ۲쵽��������________��
(2)��ӦƬ�̺н�ֹˮ��a����ʱ�ɹ۲쵽��������________________�����з�����Ӧ�����ӷ���ʽ��________________________��
(3)���Թ����еĻ���ﵹ��������У��ɹ۲쵽��������________��������Ӧ�ķ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ���ѧ�߶����ϣ����л�ѧ�Ծ������ޣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com