
œ¬ΆΦ «NaΓΔCuΓΔSiΓΔHΓΔCΓΔNΒ»‘ΣΥΊΒΞ÷ ΒΡ»έΒψΗΏΒΆΒΡΥ≥–ρΘ§Τδ÷–cΓΔdΨυ «»»ΚΆΒγΒΡΝΦ
ΒΦΧεΓΘœ¬Ν–≈–Εœ≤Μ’ΐ»ΖΒΡ «
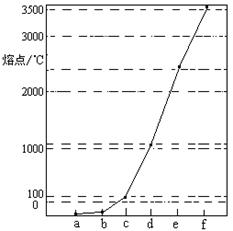
AΘ°eΓΔfΒΞ÷ ΨßΧε»έΜ· ±ΩΥΖΰΒΡ «Ι≤ΦέΦϋ
BΘ°dΒΞ÷ Ε‘”Π‘ΣΥΊ‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΘΚ1s22s22p63s23p2
CΘ°b‘ΣΥΊ–Έ≥…ΒΡΉνΗΏΦέΚ§―θΥα“Ή”κΥ°Ζ÷Ή”÷°Φδ–Έ≥…«βΦϋ
DΘ°ΒΞ÷ aΓΔbΓΔfΕ‘”ΠΒΡ‘ΣΥΊ“‘‘≠Ή”Ηω ΐ±»1ΓΟ1ΓΟ1–Έ≥…ΒΡΖ÷Ή”÷–Κ§2ΗωΠ“ΦϋΘ§2ΗωΠ–Φϋ
 ”Π”ΟΧβΧλΧλΝΖΥΡ¥®¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ
”Π”ΟΧβΧλΧλΝΖΥΡ¥®¥σ―ß≥ωΑφ…γœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
―–ΨΩΈο÷ ΒΡΈΔΙέΫαΙΙΘ§”–÷ζ”Ύ»ΥΟ«άμΫβΈο÷ ±δΜ·ΒΡ±Ψ÷ ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Δ≈OΓΔSiΓΔN‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «____________________ΓΘC60ΚΆΫπΗ’ ·ΕΦ «ΧΦΒΡΆ§ΥΊ“λ–ΈΧεΘ§Εΰ’Ώ÷–»έΒψΫœΗΏΒΡ «____________ΓΘ
ΔΤAΈΣΕΧ÷ήΤΎΫπ τ‘ΣΥΊΘ§“άΨίœ¬±μ ΐΨίΘ§AΒΡΜυΧ§‘≠Ή”ΒΡΙλΒά±μ Ψ ΫΈΣ
________________________________ΓΘ
| ΒγάκΡή/kJΓΛmolΘ≠1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
Δ«ΙΐΕ…Ϋπ τάκΉ””κΥ°Ζ÷Ή”–Έ≥…ΒΡ≈δΚœΈο «Ζώ”–―’…ΪΘ§”κΤδdΙλΒάΒΡΒγΉ”≈≈≤Φ”–ΙΊΓΘ“ΜΑψΒΊΘ§»τΈΣd0Μρd10≈≈≤Φ ±Θ§Έό―’…ΪΘΜ»τΈΣd1ΓΪd9≈≈≤Φ ±Θ§”–―’…ΪΘΜ»γ[Cu(H2O)4]2ΘΪœ‘άΕ…ΪΓΘΨί¥Υ≈–Εœ25Κ≈‘ΣΥΊMn–Έ≥…ΒΡ¬γΚœάκΉ”[Mn(H2O)6]2ΘΪ_____(ΧνΓΑ”–Γ±ΜρΓΑΈόΓ±)―’…ΪΓΘ
Δ»HΘ≠CΓ‘CΘ≠COOHΖ÷Ή”ΡΎΚ§”–ΒΡΠ“ΦϋΓΔΠ–ΦϋΒΡΗω ΐ“ά¥ΈΈΣ_______________Θ§Τδ÷–ΧΦ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ___________________ΓΘ
Δ…COΩ…“‘”κΫπ τΧζ–Έ≥…≈δΚœΈοΖ÷Ή”Fe(CO)5ΓΘFe(CO)5‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ÷ΫβΖ¥”ΠΘΚFe(CO)5(s)ΘΫFe(s)ΘΪ5CO(g)Θ§Ζ¥”ΠΙΐ≥Χ÷–Θ§ΕœΝ―ΒΡΜ·―ßΦϋ÷Μ”–≈δΈΜΦϋΘ§‘ρ–Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–Ά «______________ΓΘ
Δ W‘ΣΥΊΒΡ‘≠Ή”ΒΡMΡή≤ψΈΣ»Ϊ≥δ¬ζΉ¥Χ§Θ§«“ΚΥΆβΒΡΈ¥≥…Ε‘ΒγΉ”÷Μ”–“ΜΗωΘ§WΨßΧε÷–ΈΔΝΘΒΡΕ―ΜΐΖΫ Ϋ «œ¬ΆΦ÷– (―ΓΧνΓΑΦΉΓ±ΓΔΓΑ““Γ±ΜρΓΑ±ϊΓ±)ΘΜ»τWΨßΧε÷–“ΜΗωΨßΑϊΒΡ±Ώ≥ΛΈΣa cmΘ§‘ρWΨßΧεΒΡΟήΕ»ΈΣ Θ®–¥≥ωΚ§aΒΡ±μ¥ο ΫΘ§”ΟNA±μ ΨΑΔΖϋΦ”Β¬¬ό≥Θ ΐΘ©ΓΘ
ΦΉ ““ ±ϊ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012ΫλΫ≠Υ’ Γ»γΗό÷–―ßΗΏ»ΐœ¬―ßΤΎ÷ ΝΩΦλ≤βΜ·―ß ‘Ψμ Χβ–ΆΘΚΧνΩ’Χβ
―–ΨΩΈο÷ ΒΡΈΔΙέΫαΙΙΘ§”–÷ζ”Ύ»ΥΟ«άμΫβΈο÷ ±δΜ·ΒΡ±Ψ÷ ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Δ≈OΓΔSiΓΔN‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «____________________ΓΘC60ΚΆΫπΗ’ ·ΕΦ «ΧΦΒΡΆ§ΥΊ“λ–ΈΧεΘ§Εΰ’Ώ÷–»έΒψΫœΗΏΒΡ «____________ΓΘ
ΔΤAΈΣΕΧ÷ήΤΎΫπ τ‘ΣΥΊΘ§“άΨίœ¬±μ ΐΨίΘ§AΒΡΜυΧ§‘≠Ή”ΒΡΙλΒά±μ Ψ ΫΈΣ
________________________________ΓΘ
| ΒγάκΡή/kJΓΛmolΘ≠1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |



≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΚΰ±± ΓΨΘΟ≈ –ΗΏ»ΐ‘Σ‘¬ΒςΩΦάμΉέΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
ΔώΘ°―ΊΚΘΒΊ«χ”–Ή≈ΖαΗΜΒΡΚΘΥ°Ή ‘¥Θ§ΚΘΥ°÷–÷ς“ΣΚ§”–NaΘΪΓΔKΘΪΓΔCa2ΘΪΓΔMg2ΘΪΓΔClΘ≠ΓΔSO42Θ≠ΓΔBrΘ≠ΓΔCO32Θ≠ΓΔHCO3Θ≠Β»άκΉ”ΓΘΚœάμάϊ”ΟΉ ‘¥ΚΆ±ΘΜΛΜΖΨ≥ «Ω…≥÷–χΖΔ’ΙΒΡ÷Ί“Σ±Θ÷ΛΓΘ
 Θ®1Θ©ΚΘΥ°Ψ≠Ιΐ¥ΠάμΚσΩ…“‘ΒΟΒΫΈόΥ°¬»Μ·ΟΨΘ§ΈόΥ°¬»Μ·ΟΨ «ΙΛ“Β÷Τ»ΓΟΨΒΡ‘≠ΝœΓΘ ‘–¥≥ωΒγΫβ»έ»Ύ¬»Μ·ΟΨ÷Τ»ΓΫπ τΟΨΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ???????????????????????????????? ΓΘ
Θ®1Θ©ΚΘΥ°Ψ≠Ιΐ¥ΠάμΚσΩ…“‘ΒΟΒΫΈόΥ°¬»Μ·ΟΨΘ§ΈόΥ°¬»Μ·ΟΨ «ΙΛ“Β÷Τ»ΓΟΨΒΡ‘≠ΝœΓΘ ‘–¥≥ωΒγΫβ»έ»Ύ¬»Μ·ΟΨ÷Τ»ΓΫπ τΟΨΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ Ϋ???????????????????????????????? ΓΘ
Θ®2Θ©Ρ≥Μ·ΙΛ≥ß…ζ≤ζΙΐ≥Χ÷–Μα≤ζ…ζΚ§”–Cu2ΘΪΚΆPb2ΘΪΒΡΈέΥ°ΓΘ≈≈Ζ≈«ΑΡβ”Ο≥ΝΒμΖ®≥ΐ»Ξ’βΝΫ÷÷άκΉ”Θ§ΗυΨίœ¬Ν– ΐΨίΘ§Ρψ»œΈΣΆΕ»κ?????????? Θ®―ΓΧνΓΑNa2SΓ±ΜρΓΑNaOHΓ±Θ©–ßΙϊΗϋΚΟΓΘ
Ρ―»ήΒγΫβ÷ | Cu(OH)2 | CuS | Pb(OH)2 | PbS |
Ksp | 4Θ°8ΓΝ10Θ≠20 | 6Θ°3ΓΝ10Θ≠36 | 1Θ°2ΓΝ10Θ≠15 | 1Θ°0ΓΝ10Θ≠28 |
Θ®3Θ©ΜπΝΠΖΔΒγ‘ΎΈ“ΙζΒΡΡή‘¥άϊ”Ο÷–’ΦΫœ¥σ±»÷ΊΘ§ΒΪ «≈≈Ζ≈≥ωΒΡSO2Μα‘λ≥…“ΜœΒΝ–ΜΖΨ≥ΚΆ…ζΧ§Έ ΧβΓΘάϊ”ΟΚΘΥ°Ά―Νρ «“Μ÷÷”––ßΒΡΖΫΖ®Θ§ΤδΙΛ“’Νς≥Χ»γœ¬ΆΦΥυ ΨΘΚ

ΔΌΧλ»ΜΚΘΥ°ΒΡpHΓ÷8Θ§ ‘”ΟάκΉ”ΖΫ≥Χ ΫΫβ ΆΧλ»ΜΚΘΥ°≥ »θΦν–‘ΒΡ‘≠“ρ????????? Θ®»Έ–¥“ΜΗωΘ©ΓΘ
ΔΎΡ≥―–ΨΩ–ΓΉιΈΣΧΫΨΩΧαΗΏΚ§Νρ―ΧΤχ÷–SO2Έϋ ’–߬ ΒΡ¥κ ©Θ§Ϋχ––ΝΥΧλ»ΜΚΘΥ°Έϋ ’Κ§Νρ―ΧΤχΒΡΡΘΡβ Β―ιΘ§ Β―ιΫαΙϊ»γΆΦΥυ ΨΓΘ

«κΡψΗυΨίΆΦ Ψ Β―ιΫαΙϊΘ§ΨΆ»γΚΈΧαΗΏ“ΜΕ®≈®Ε»Κ§Νρ―ΧΤχ÷–SO2ΒΡΈϋ ’–߬ Θ§Χα≥ω“ΜΧθΚœάμΜ·Ϋ®“ιΘΚ??? ΓΘ
ΔέΧλ»ΜΚΘΥ°Έϋ ’ΝΥΚ§Νρ―ΧΤχΚσΜα»ή”–H2SO3ΓΔHSO3Θ≠Β»Ζ÷Ή”ΜράκΉ”Θ§ Ι”Ο―θΤχΫΪΤδ―θΜ·ΒΡΜ·―ß‘≠άμ «?????????????????? Θ®»Έ–¥“ΜΗωΜ·―ßΖΫ≥Χ ΫΜράκΉ”ΖΫ≥Χ ΫΘ©ΓΘ―θΜ·ΚσΒΡΓΑΚΘΥ°Γ±–η“Σ“ΐ»κ¥σΝΩΒΡΧλ»ΜΚΘΥ°”κ÷°ΜλΚœΚσ≤≈Ρή≈≈Ζ≈Θ§ΗΟ≤ΌΉςΒΡ÷ς“ΣΡΩΒΡ «???????????????????????? ΓΘ
ΔρΘ°Ρή‘¥ «»Υάύ…ζ¥φΚΆΖΔ’ΙΒΡ÷Ί“Σ÷ß÷υΓΘ―–ΨΩΜ·―ßΖ¥”ΠΙΐ≥Χ÷–ΒΡΡήΝΩ±δΜ·‘ΎΡή‘¥Ϋτ»±ΒΡΫώΧλΨΏ”–÷Ί“ΣΒΡάμ¬έ“β“εΓΘ“―÷Σœ¬Ν–»»Μ·―ßΖΫ≥Χ Ϋ
ΔΌ2H2(g)+O2(g)ΘΫ2H2O(l)????  HΘΫΘ≠570kJ/molΘΜ
HΘΫΘ≠570kJ/molΘΜ
ΔΎH2(g)+1/2O2(g)ΘΫH2O(g)???  HΘΫΘ≠242kJ/molΘΜ
HΘΫΘ≠242kJ/molΘΜ
ΔέC(s)+1/2O2(g)ΘΫCO(g)????  HΘΫΓΣ110Θ°5kJ/moLΘΜ
HΘΫΓΣ110Θ°5kJ/moLΘΜ
ΔήC(s)+O2(g)ΘΫCO2(g)???????  HΘΫΓΣ393Θ°5kJ/moLΘΜ
HΘΫΓΣ393Θ°5kJ/moLΘΜ
ΔίCO2(g)+2H2O(g)ΘΫ2CH4(g)+2 O2(g)?  HΘΫ+890kJ/moL
HΘΫ+890kJ/moL
ΜΊ¥πœ¬Ν–Έ Χβ
Θ®1Θ©…œ ωΖ¥”Π÷– τ”ΎΈϋ»»Ζ¥”ΠΒΡ «??????????????? ΓΘ
Θ®2Θ©H2ΒΡ»Φ…’»»ΈΣΓςHΘΫ??????????????? ΓΘ
Θ®3Θ©Η«ΥΙΕ®¬…‘Ύ…ζ≤ζΚΆΩΤ―ß―–ΨΩ÷–”–Κή÷Ί“ΣΒΡ“β“εΓΘ”––©Ζ¥”ΠΒΡΖ¥”Π»»Υδ»ΜΡ―÷±Ϋ”≤βΕ®Θ§ΒΪΩ…Ά®ΙΐΦδΫ”ΒΡΖΫΖ®«σΒΟΓΘ“―÷ΣC(s) + H2O(g)ΘΫH2(g)+ CO(g)????  HΘΫakJ/moLΘΜ‘ρaΘΫ???????? ΘΜΗΟΖ¥”ΠΒΡλΊ
HΘΫakJ/moLΘΜ‘ρaΘΫ???????? ΘΜΗΟΖ¥”ΠΒΡλΊ S???????? 0(―ΓΧνΓΑΘΨΓ±ΓΔΓΑΘΫΓ±ΓΔΓΑΘΦΓ±)ΘΜ“―÷ΣΉ‘”…Ρή
S???????? 0(―ΓΧνΓΑΘΨΓ±ΓΔΓΑΘΫΓ±ΓΔΓΑΘΦΓ±)ΘΜ“―÷ΣΉ‘”…Ρή GΘΫ
GΘΫ HΓΣT
HΓΣT SΘ§Β±
SΘ§Β± GΘΦ0 ±Ω…Ή‘ΖΔΫχ––ΓΘ‘ρΗΟΖ¥”Π‘Ύ ≤Ο¥ΧθΦΰœ¬Ω…Ή‘ΖΔΫχ––__________________ΓΘ
GΘΦ0 ±Ω…Ή‘ΖΔΫχ––ΓΘ‘ρΗΟΖ¥”Π‘Ύ ≤Ο¥ΧθΦΰœ¬Ω…Ή‘ΖΔΫχ––__________________ΓΘ
Θ®4Θ©COΖ÷Έω“«“‘»ΦΝœΒγ≥ΊΈΣΙΛΉς‘≠άμΘ§ΤδΉΑ÷Ο»γΆΦΥυ ΨΘ§ΗΟΒγ≥Ί÷–ΒγΫβ÷ ΈΣ―θΜ·νΤΘ≠―θΜ·ΡΤΘ§Τδ÷–O2-Ω…“‘‘ΎΙΧΧεΫι÷ NASICON÷–Ή‘”…“ΤΕ·ΓΘœ¬Ν–ΥΒΖ®¥μΈσΒΡ «????? ΓΘ

AΘ°ΗΚΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣΘΚCO+O2ΓΣ®D2e-ΘΫCO2
BΘ°ΙΛΉς ±ΒγΦΪbΉς’ΐΦΪΘ§O2ΓΣ”…ΒγΦΪaΝςœρΒγΦΪb
CΘ°ΙΛΉς ±ΒγΉ””…ΒγΦΪaΆ®Ιΐ¥ΪΗ–ΤςΝςœρΒγΦΪb
DΘ°¥ΪΗ–Τς÷–Ά®ΙΐΒΡΒγΝς‘Ϋ¥σΘ§Έ≤Τχ÷–COΒΡΚ§ΝΩ‘ΫΗΏ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξ…ΫΕΪ ΓΝΌ“ –ΗΏ»ΐ5‘¬ΗΏΩΦΡΘΡβάμΉέΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Ϋπ τΆ≠ΙψΖΚΒΊ”Π”Ο”ΎΒγΤχΓΔΜζ–Β÷Τ‘λΓΔΙζΖάΒ»Νλ”ρΓΘ
Θ®1Θ©Cu «‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎ29Κ≈‘ΣΥΊΘ§–¥≥ωΒΎ»ΐ÷ήΤΎΜυΧ§‘≠Ή”Έ¥≥…Ε‘ΒγΉ” ΐ”κCuœύΆ§«“ΒγΗΚ–‘Ήν¥σΒΡ‘ΣΥΊ « (Χν‘ΣΥΊΟϊ≥Τ)ΓΘ
Θ®2Θ©CuO ή»»“ΉΖ÷ΫβΈΣCu2OΚΆO2Θ§«κ¥”Ά≠ΒΡ‘≠Ή”ΫαΙΙά¥ΥΒΟςCuO ή»»“ΉΖ÷ΫβΒΡ‘≠“ρΘΚ
ΓΘ
Θ®3Θ©œ¬ΆΦ «Ά≠ΒΡΡ≥÷÷―θΜ·ΈοΒΡΨßΑϊ Ψ“βΆΦΓΘ“―÷ΣΗΟΨßΑϊΒΡ±Ώ≥ΛΈΣa cmΘ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΈΣNAΘ§ΗΟΨßΧεΒΡΟήΕ»ΈΣ ΓΘ

Θ®4Θ©œρΝρΥαΆ≠»ή“Κ÷–ΒΈΦ”Α±Υ°Μα…ζ≥…άΕ…Ϊ≥ΝΒμΘ§‘ΎΒΈΦ”Α±Υ°ΒΫ≥ΝΒμΗ’ΚΟ»Ϊ≤Ω»ήΫβΩ…ΒΟΒΫ…νάΕ…Ϊ»ή“ΚΘ§ΦΧ–χœρΤδ÷–Φ”»κΦΪ–‘Ϋœ–ΓΒΡ““¥ΦΩ…“‘…ζ≥……νάΕ…ΪΒΡ[Cu(NH3)4]SO4ΓΛH2O≥ΝΒμΓΘ
ΔΌSO42Θ≠÷–S‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ ΓΘ
ΔΎNH3Ζ÷Ή”ΡΎΒΡHΓΣNΓΣHΦϋΫ«ΓΓΓΓΓΓΓΓ Θ®ΧνΓΑ¥σ”ΎΓ±ΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±Θ©H2OΖ÷Ή”ΡΎΒΡHΓΣOΓΣHΦϋΫ«ΓΘ
ΔέSΓΔNΓΔO»ΐ÷÷‘ΣΥΊΒΎ“ΜΒγάκΡή”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2012―ßΡξΫ≠Υ’ ΓΗΏ»ΐœ¬―ßΤΎ÷ ΝΩΦλ≤βΜ·―ß ‘Ψμ Χβ–ΆΘΚΧνΩ’Χβ
―–ΨΩΈο÷ ΒΡΈΔΙέΫαΙΙΘ§”–÷ζ”Ύ»ΥΟ«άμΫβΈο÷ ±δΜ·ΒΡ±Ψ÷ ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Δ≈OΓΔSiΓΔN‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «____________________ΓΘC60ΚΆΫπΗ’ ·ΕΦ «ΧΦΒΡΆ§ΥΊ“λ–ΈΧεΘ§Εΰ’Ώ÷–»έΒψΫœΗΏΒΡ «____________ΓΘ
ΔΤAΈΣΕΧ÷ήΤΎΫπ τ‘ΣΥΊΘ§“άΨίœ¬±μ ΐΨίΘ§AΒΡΜυΧ§‘≠Ή”ΒΡΙλΒά±μ Ψ ΫΈΣ
________________________________ΓΘ
|
ΒγάκΡή/kJΓΛmolΘ≠1 |
I1 |
I2 |
I3 |
I4 |
|
A |
932 |
1821 |
15390 |
21771 |
Δ«ΙΐΕ…Ϋπ τάκΉ””κΥ°Ζ÷Ή”–Έ≥…ΒΡ≈δΚœΈο «Ζώ”–―’…ΪΘ§”κΤδdΙλΒάΒΡΒγΉ”≈≈≤Φ”–ΙΊΓΘ“ΜΑψΒΊΘ§»τΈΣd0Μρd10≈≈≤Φ ±Θ§Έό―’…ΪΘΜ»τΈΣd1ΓΪd9≈≈≤Φ ±Θ§”–―’…ΪΘΜ»γ[Cu(H2O)4]2ΘΪœ‘άΕ…ΪΓΘΨί¥Υ≈–Εœ25Κ≈‘ΣΥΊMn–Έ≥…ΒΡ¬γΚœάκΉ”[Mn(H2O)6]2ΘΪ_____(ΧνΓΑ”–Γ±ΜρΓΑΈόΓ±)―’…ΪΓΘ
Δ»HΘ≠CΓ‘CΘ≠COOHΖ÷Ή”ΡΎΚ§”–ΒΡΠ“ΦϋΓΔΠ–ΦϋΒΡΗω ΐ“ά¥ΈΈΣ_______________Θ§Τδ÷–ΧΦ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ___________________ΓΘ
Δ…COΩ…“‘”κΫπ τΧζ–Έ≥…≈δΚœΈοΖ÷Ή”Fe(CO)5ΓΘFe(CO)5‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ÷ΫβΖ¥”ΠΘΚFe(CO)5(s)ΘΫFe(s)ΘΪ5CO(g)Θ§Ζ¥”ΠΙΐ≥Χ÷–Θ§ΕœΝ―ΒΡΜ·―ßΦϋ÷Μ”–≈δΈΜΦϋΘ§‘ρ–Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–Ά «______________ΓΘ
Δ W‘ΣΥΊΒΡ‘≠Ή”ΒΡMΡή≤ψΈΣ»Ϊ≥δ¬ζΉ¥Χ§Θ§«“ΚΥΆβΒΡΈ¥≥…Ε‘ΒγΉ”÷Μ”–“ΜΗωΘ§WΨßΧε÷–ΈΔΝΘΒΡΕ―ΜΐΖΫ Ϋ «œ¬ΆΦ÷– (―ΓΧνΓΑΦΉΓ±ΓΔΓΑ““Γ±ΜρΓΑ±ϊΓ±)ΘΜ»τWΨßΧε÷–“ΜΗωΨßΑϊΒΡ±Ώ≥ΛΈΣa cmΘ§‘ρWΨßΧεΒΡΟήΕ»ΈΣ Θ®–¥≥ωΚ§aΒΡ±μ¥ο ΫΘ§”ΟNA±μ ΨΑΔΖϋΦ”Β¬¬ό≥Θ ΐΘ©ΓΘ



ΦΉ ““ ±ϊ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com