
��CH4����ԭNOx�������������������Ⱦ����֪CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)����H����574 kJ·mol��1��CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)����H����1 160 kJ·mol��1�����ڱ�״����4.48 L CH4ǡ���ܽ�һ����NO2��ԭ��N2��H2O(g)�������������зų�������Ϊ(����)
A��114.8 kJ B��232 kJ
C��368.8 kJ D��173.4 kJ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬһ״̬�£�20 mL A2������30 mL B2����ǡ����ȫ��Ӧ����20 mLij����X����X�Ļ�ѧʽΪ(����)
A��A2B3 B��AB2
C��AB3 D��A3B2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����Ҳ����KMnO4�����Ũ���ᷴӦ����������Ӧ����ʽ���£�2KMnO4��16HCl(Ũ)===2KCl��2MnCl2��8H2O��5Cl2�����ش��������⣺
(1)�÷�Ӧ����������__________����ԭ������____________��
(2)���μӷ�Ӧ�ĸ������Ϊ7.9 g����������HCl�����ʵ����Ƕ��٣������ɵ�����ȫ��ͨ����������������Һ�У����ɴ������ƶ��ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
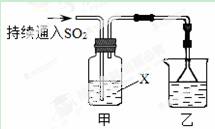 ij��ѧ��ȤС��������װ��̽��SO2��ijЩ��ѧ���ʡ�
ij��ѧ��ȤС��������װ��̽��SO2��ijЩ��ѧ���ʡ�
��1��װ���ҵ������� ��
��2����XΪƷ����Һ���۲쵽��Һ��ɫ��˵��SO2���� ������ţ���ͬ������XΪNa2S��Һ���۲쵽��Һ�г��ֵ���ɫ���ǣ�˵��SO2���� ��
a�������� b����ԭ�� c��Ư����
��3�����Լ�XΪCa(ClO)2��Һ���ɹ۲쵽��ɫ�������ɣ���ɸù��̵����ӷ���ʽ��
 Ca2��+
Ca2��+ ClO��+
ClO��+ SO2+
SO2+ H2O��
H2O�� ��+
��+ Cl��+
Cl��+ SO42��+
SO42��+ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
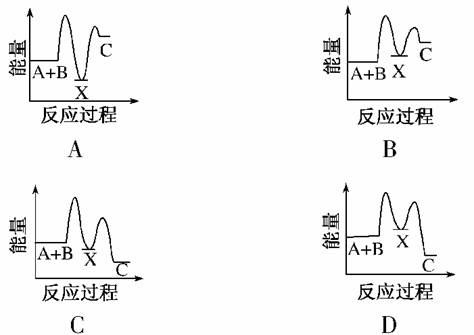
��ӦA��B����C(��H<0)���������У�
��A��B����X(��H>0)����X����C(��H<0)��
����ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����(����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaCO3(s)===CaO��CO2(g)����H��177.7 kJ
��C(s)��H2O(s)===CO(g)��H2(g)
��H����131.3 kJ/mol
��H2SO4(l)��NaOH(l)===Na2SO4(l)��H2O(l)
��H����57.3 kJ/mol
��C(s)��O2(g)===CO2(g)����H����393.5 kJ/mol
��CO(g)��O2(g)===CO2(g)����H����283 kJ/mol
��HNO3(aq)��NaOH(aq)===NaNO3(aq)��H2O(l)
��H����57.3 kJ/mol
��2H2(g)��O2(g)===2H2O(l)����H����517.6 kJ/mol
(1)�����Ȼ�ѧ����ʽ�У�����ȷ����________������ȷ�����ɷֱ���_______________________________________________________________��
(2)����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽ_____________��
(3)������Ӧ�У���ʾȼ���ȵ��Ȼ�ѧ����ʽ��________________����ʾ�к��ȵ��Ȼ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Fe2(SO4)3��CuSO4��ϡH2SO4�Ļ��Һ�м���Fe�ۣ���Ӧ��������ʣ��Ĺ����˳����ܱ�������������Ӧ����Һ�д��ڽ϶����������
A��Fe3�� B��Cu2��
C��Fe2�� D��H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A����ѧ�仯������ԭ�ӵ�������Ϲ���
B�����ݻ�ѧ��Ӧ�е������仯�������ѧ��Ӧ�ɷ�Ϊ���ȷ�Ӧ�ͷ��ȷ�Ӧ
C����ѧ��Ӧ�е������仯�������䷴Ӧ�������й�
D����ѧ��Ӧ�е������仯������������ʽ���ֳ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�������������ʵ����淽������ȷ���ǣ� ��
A��Ũ�����ô�������ϸ�ڡ���ɫ�Լ�ƿʢ�ţ���������������
B�����������Ʊ�����ú����
C������Һ�����ˮ��棬��ֹ��ӷ�
D����������������Һʱ��Ҫ�����м����������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com