
��12�֣����ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ��
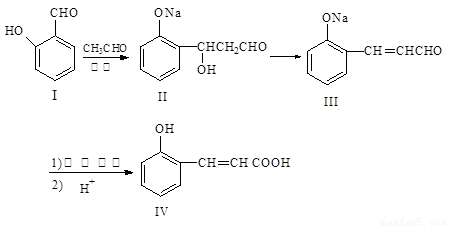
�Իش��������⣺
��1��������III ��������Һ�з�����Ӧ��ѧ����ʽ: ����2�֣�
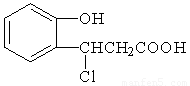
��2���л��� X Ϊ������IV��ͬ���칹�壬��֪�л���X �������ص㣺
���DZ��Ķ�λȡ���������NaHCO3 ��Ӧ�ų����壬���ܷ���������Ӧ��
��д��������X�Ľṹ��ʽ ����4�֣�
��3������˵����ȷ���� ����4�֣�
A.������I���Ȼ�����Һ����ɫ B.������II����NaHCO3��Һ��Ӧ
C.1mol������IV��ȫȼ������ 9.5molO2 D.1mol������III�������3 molH2 ��Ӧ
��4���л���R��C9H9ClO3��������ӦҲ���Ƶû�����IV���� R ��NaOH ����Һ�з�Ӧ�Ļ�ѧ����ʽΪ ����2�֣�
��1����2�֣�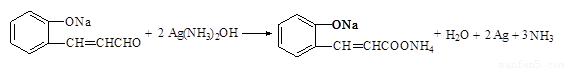 ��2��
��2��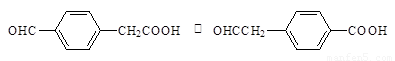
��3��A ��C ��4 �֣�
��4����2 �֣�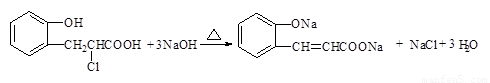 ��
��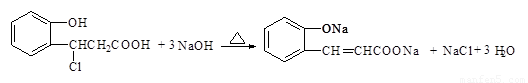
����������1��������III�к���ȩ�����ܷ���������Ӧ������ʽ��

��2������NaHCO3 ��Ӧ�ų����壬˵�������Ȼ����ܷ���������Ӧ��������ȩ��������Ϊ���DZ��Ķ�λȡ������Խṹ��ʽ��
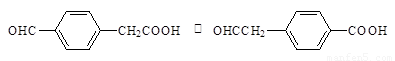
��3��������I�к��з��ǻ�������ѡ��A��ȷ��������II��û���Ȼ���������NaHCO3��Һ��Ӧ��ѡ��B����ȷ�����ݻ�����IV�ķ���ʽ��֪��1mol������IV��ȫȼ������ 9.5molO2��ѡ��C��ȷ��������III�к���1��̼̼˫����1��ȩ����1��������1mol������III�������5 molH2 ��Ӧ��ѡ��D����ȷ����ѡAC��
��4���л���R��C9H9ClO3��������ӦҲ���Ƶû�����IV���� R�Ľṹ��ʽ��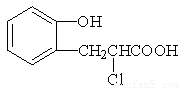 ��
�� �����Է�Ӧ�ķ���ʽ��
�����Է�Ӧ�ķ���ʽ��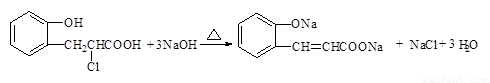 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





 ��
��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫��ɽ������������ѧ�߶���һѧ�����ƻ�ѧ�Ծ����������� ���ͣ������
��12�֣����ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ��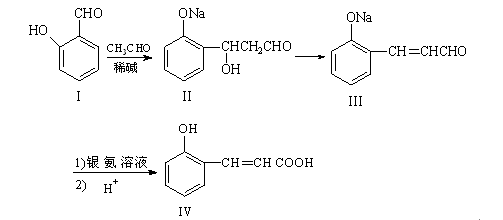
�Իش��������⣺
��1��������III ��������Һ�з�����Ӧ��ѧ����ʽ: ����2�֣�
��2���л��� X Ϊ������IV��ͬ���칹�壬��֪�л���X �������ص㣺
���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ��
��д��������X�Ľṹ��ʽ ����4�֣�
��3������˵����ȷ���� ����4�֣�
| A��������I���Ȼ�����Һ����ɫ | B��������II����NaHCO3��Һ��Ӧ |
| C��1mol������IV��ȫȼ������ 9.5molO2 | D��1mol������III�������3 molH2 ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ����11�¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
���ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ
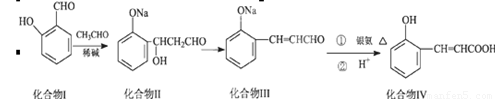
�Իش��������⣺
��1��������I��CH3CHO���������л���Ӧ������
��2��������III��������Һ�з�����Ӧ��ѧ����ʽ
��3������˵����ȷ���ǣ�˫ѡ��
A��������I���Ȼ�����Һ����ɫ
B��������II����NaHCO3��Һ��Ӧ
C��1mol������IV��ȫȼ������9.5molO2
D��1mol������III����3 mol H2��Ӧ
��4���л���XΪ������IV��ͬ���칹�壬��֪�л���X�������ص㣺
���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ��
��д��������X�Ľṹ��ʽ ��д�����֣�
��5���л���R����ͼ��������ӦҲ���Ƶû�����IV����R��NaOH����Һ�з�Ӧ�Ļ�ѧ����ʽΪ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����з�خ������ͳ����һ�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
���ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ��
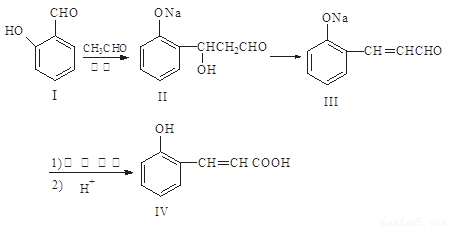
�Իش��������⣺
��1��������II��������III���л���Ӧ���ͣ� ��
��2��������III��������Һ�з�����Ӧ��ѧ����ʽ�� ��
��3���л���XΪ������IV��ͬ���칹�壬��֪�л���X�������ص㣺
���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ��
��д��������X�Ľṹ��ʽ_____________________��___________________________
��4������˵����ȷ���� ��
A��������I���Ȼ�����Һ����ɫ
B��������II����NaHCO3��Һ��Ӧ
C��1mol������IV��ȫȼ������ 9.5molO2
D��1mol������III�������3 molH2 ��Ӧ
��5��������IV������NaOH��Һ��Ӧ���Ƶû�����V���л���R��C9H9ClO3����NaOH ����Һ�з�ӦҲ���Ƶû�����V����д���û�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com