

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����Ӧ��ϵȫ����ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.����ˮ��ͨ������Cl2��������ɱ��
B.���״���ϴ��ˮƿ���ڱڸ��ŵ�ˮ��(��CaCO3)
C.��SO2Ư��ʳƷ
D.��С�մ�(NaHCO3)�������ţ�������ͷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ���Իش�
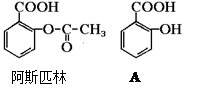
��1����˹ƥ�ֿɿ����������ʣ��ڷ�����θ�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B���ֲ������A�Ľṹ��ʽ��ͼ����B�Ľṹ��ʽΪ�� ��A�еĺ��������������ǣ� �� ��
��2����˹ƥ�ָ�С�մ�(NaHCO3)ͬʱ���ã���ʹ����ˮ�����A��С�մ�Ӧ�����ɿ�����������Һ�ų������εĽṹ��ʽΪ�� ��
��3������ˮ�����A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��
��
��4������ˮ�����A ��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ���Իش�
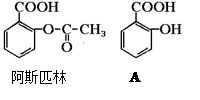
��1����˹ƥ�ֿɿ����������ʣ��ڷ�����θ�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B���ֲ������A�Ľṹ��ʽ��ͼ����B�Ľṹ��ʽΪ�� ��A�еĺ��������������ǣ� �� ��
��2����˹ƥ�ָ�С�մ�(NaHCO3)ͬʱ���ã���ʹ����ˮ�����A��С�մ�Ӧ�����ɿ�����������Һ�ų������εĽṹ��ʽΪ�� ��
��3������ˮ�����A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��
��
��4������ˮ�����A ��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ������ޣ� ���ͣ������
����Ӫ��ƽ�⣬����ʹ��ҩ���DZ�֤���Ľ��������������������Ч�ֶΡ�
��1����2�֣� ��������������Ҫ���ࡢ �� ά���ء�ˮ��������(�������)��������Ӫ�����ʡ�
�ڣ�3�֣���˾ƥ�־���
���á����ڴ������ð�˾ƥ�֣���ˮ�����ˮ����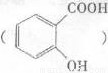 �ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�(NaHCO3)��Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⡣д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
��
�ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�(NaHCO3)��Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⡣д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
��
��2����2�֣���������������ˮ������ղ����ǰ����ᡣ������ͼ���߷�����д���ʵ��Ĺ����ŷ��ţ����������ͨʽ����������
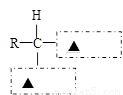
��3������ˮ������ղ����� ��Ҫ������۵ĵ���ø�������Ѿ�������ˮ�⣬��ȡ����������Һ�����Һ�������ԣ��ټ��� �����Լ������ƣ������Ⱥ��ٸ���ʵ�������жϣ���Ҫ�������û����ȫˮ��ģ���ȡ����������Һ���뼸�� ��Һ��Ӧ�۲쵽������ɫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com