
���� ��������=���Ӳ���������Ԫ����������������������������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬C��Mg��Ԫ���γ�����������������Ϊ1��1�����ӻ�����ΪMgC2������ˮ��Ӧ������Ȳ��
��� �⣺Ԫ��þ��������Ϊ12����ԭ�ӻ�̬ʱ�ĺ�������Ų�ʽΪ1s22s22p63s2��Ԫ�غ����������Ӳ㣬�����2�����ӣ���������=���Ӳ���������Ԫ������������������������������λ��Ϊ��3���ڵڢ�A�壬����þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵ�ʱ��Ҫ��������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬C��Mg��Ԫ���γ�����������������Ϊ1��1�����ӻ�����ΪMgC2����ˮ��Ӧ���ɵ�����ΪC2H2��������ĽṹʽΪH-C��C-H��
�ʴ�Ϊ����3���ڵڢ�A�壻Ӳ�ȴ���ʴ���ܶ�С��H-C��C-H��
���� ���⿼����Ԫ�������ڱ���λ�õ��жϡ��Ͻ�����ʡ�̼��þ�����ʵȣ���ȷԪ��ԭ�Ӻ�����Ӳ�����������������������������������֮��Ĺ�ϵ�����ճ����Ͻ�������Լ�������Ϣ�ƶ�̼��þ��ˮ������ǽ����Ĺؼ������ض�ѧ���������⡢֪ʶǨ�������Ŀ��飬��Ŀ�Ѷ��еȣ�


 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ�� | �����Ϣ |
| X | XԪ�ؿ��γ���Ȼ����Ӳ���������� |
| Y | �䵥��Ϊ˫ԭ�ӷ��ӣ�������⻯���ˮ��Һ��ʹ��̪��� |
| Z | Z�Ƕ�������������ʧȥ���ӵ�Ԫ�� |
| M | M��һ��ͬλ�ص�������Ϊ34��������Ϊ18 |
| N | N�Ǿ����Ϻ�ɫ����Ľ������кܺõ���չ�ԡ������Ժ͵����� |
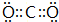 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��Ļ�ѧʽ ���볣��K | ������ H2S | ������ H2SO3 | ���� H2CrO4 | ���� HCN |
| K1 | 9.1��10-8 | 1.5��10-2 | 1.8��10-1 | 5.0��10-10 |
| K2 | 1.1��10-12 | 1.0��10-7 | 3.2��10-7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
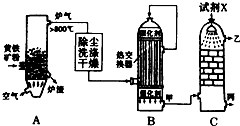 �ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺
�ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��CaO��Ca Cl2 | B�� | O2��CuO��Cu��OH��2 | ||
| C�� | C��CO2��Na2CO3 | D�� | NaOH��Na2CO3��NaCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com