
���������У�500��ʱ��NH3��O2���ܷ������·�Ӧ��
��4NH3(g)��5O2(g) 4NO(g)��6H2O(g)��H����9072kJ/mol K��1.1��1026
4NO(g)��6H2O(g)��H����9072kJ/mol K��1.1��1026
��4NH3(g)��4O2(g) 2N2O(g)��6H2O(g)��H����1104.9kJ/mol K��4.4��1028
2N2O(g)��6H2O(g)��H����1104.9kJ/mol K��4.4��1028
��4NH3(g)��3O2(g) 2N2(g)��6H2O(g)��H����1269.02kJ/mol K��7.1��1034
2N2(g)��6H2O(g)��H����1269.02kJ/mol K��7.1��1034
���У��ڡ����Ǹ���Ӧ����Ҫ���ٸ���Ӧ����ߵ�λʱ����NO�IJ��ʣ�������Ĵ�ʩ��()
A������O2Ũ�� B��ʹ�ú��ʵĴ��� C����Сѹǿ D�������¶�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�����ɹų����и������Ĵ�ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��Ԫ�����������������й㷺��Ӧ��
��1��Pԭ�Ӽ۵����Ų�ͼΪ_________��
��2����(�������)�ٷ��ӽṹ����ͼ1:
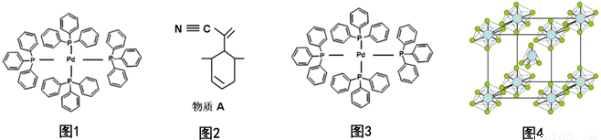
Pԭ���������������̬Χ������ԭ�������ϣ���ԭ�ӵ��ӻ��������Ϊ_________���жϸ�������ˮ���ܽ�Ȳ����Խ���_________�������ʿ�����ͼ2��ʾ����A�ĺϳɣ�
����A��̼ԭ���ӻ��������Ϊ _________��һ��A����������̼ԭ����ĿΪ _________��
��3����ͼ3�б�ʾ����(�������)�ٷ�������λ����
��4��PCl5��һ�ְ�ɫ���壬�ں����ܱ������м��ȿ���148��Һ�����γ�һ���ܵ�������壬������к���һ�����������������Ӻ�һ�����������������ӣ�������P-Cl�ļ���ֻ��198nm��206nm���֣����������ӵĻ�ѧʽΪ_________�������������������м���С��PCl3�ļ���ԭ��Ϊ_________���þ���ľ�������ͼ4��ʾ��������ľ����߳�Ϊa pm��NAΪ����٤��������ֵ����þ�����ܶ�Ϊ_________g/cm3��
��5��PBr5��̬���ӵĽṹ��PCl5���ƣ���������Ҳ�ܵ��磬���ⶨ֪����ֻ����һ��P-Br���������õ��뷽��ʽ����PBr5�����ܵ����ԭ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��I-IV��ʵ������Ԥ����ȷ����( )
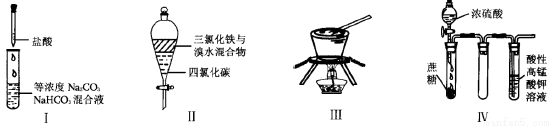
A��ʵ��I:��εμ�����ʱ���Թ�������������������.
B��ʵ��II:�������,�²���ҺΪ�Ⱥ�ɫ���ϲ���ɫ
C��ʵ��III:�ӱ���ʳ��ˮ����ȡNaCl����
D��װ��IV:����KMnO4��Һ�������ݳ��֣�����Һ��ɫ����dz������ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�µ���ѧ�����ʾ��ȷ����
A��H2O2�ĵ���ʽ��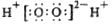
B��CH3CH2NO2��H2NCH2COOH��Ϊͬ���칹��
C��������ṹ��ʽ��CH2ClCH2Cl
D��C2H4��C3H6һ����Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й��ڸ�ͼ��Ľ��ͻ���۲���ȷ����
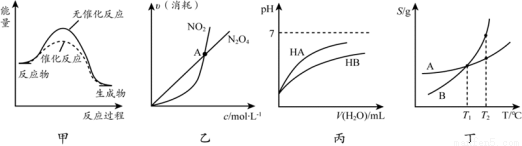
A���ɼ�֪��ʹ�ô�����Ӱ�췴Ӧ��
B�����ҿ�֪�����ں��º��������µķ�Ӧ2NO2 (g)  N2O4 (g)��A��Ϊƽ��״̬
N2O4 (g)��A��Ϊƽ��״̬
C���ɱ���֪��ͬ�¶ȡ�ͬŨ�ȵ�NaA��Һ��NaB��Һ��ȣ���pHǰ��С�ں���
D���ɶ���֪����T1 �� ��A��B������Һ������T2 ��ʱ��A��B��Һ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���з���������ԭ�Ӷ���������� 8 ���ӽṹ����( )
��SF6 ��PCl5 ��PCl3 ��CS2 ��NO2 ��N2
A���٢ڢܢ� B���ڢۢݢ� C���ۢܢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�캣��ʦ���и����ھŴ��¿���ѧ�Ծ��������棩 ���ͣ������
ij�С�����̼�����Ƶ��Ʊ�ʵ�顣�йط�Ӧ�Ļ�ѧ����ʽΪ��
NH3��CO2��H2O=NH4HCO3 ��NH4HCO3��NaCl=NaHCO3����NH4Cl
��1��һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ������ͼ��ʾ(ͼ�мг֡��̶��õ�����δ����)���Իش������й����⣺
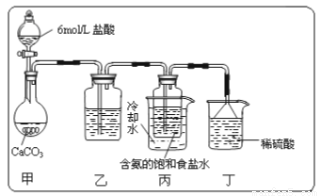
��ʵ�����ư����Ļ�ѧ����ʽ__________________��
����װ���е��Լ���______����װ����ϡ�����������____________��
��ʵ����������NaHCO3����IJ�����____________(��������������)���ò�������Ҫ�IJ��������У���������____________��
��2��̼�������������ù���12.28g ��������ʯ��ˮ��ַ�Ӧ�����ó�����ϴ�ӡ��������Ϊ 12.00g,�����ù�����̼���Ƶ���������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�캣����ѧ�����߿�ģ��Ż�ѧ�Ծ��������棩 ���ͣ�ʵ����
����ʯ(һ�ຬ�Ƶ������ο����ܳ�)ԭ��������淋Ĺ�������ͼ���£�
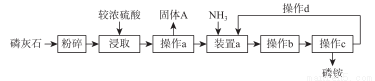
��֪�������������Ϊ��ɫ����,����������һ����ȶ��������林�֮��������鱗��ȶ�������������ʹ�á�
��1���������ȡ��ʯ�������������__________�����ʡ�
��2������ a ��������__________��װ��a��Ҫ���ơ�N/P����ʹ��Ӧ�����ɽ϶��һ����ʽ�Σ��仯ѧʽΪ__________��
��3������ A �Ļ�ѧʽΪ__________������;��__________��
��4������bΪ__________��__________��
��5���������________�� (��д����һ�����ϡ�)
��6������CΪ���ˡ�ϴ�ӡ������ϴ�ӹ����п�ѡ�������Լ�����ϴ��________ (ѡ����ĸ)�����ŵ���________��
��7�������������в��� d ��Ŀ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�캣����ѧ�����߿�ģ��˻�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʽΪ C8H8��ij�л���,����ʹ���Ը��������Һ��ɫ��Ҳ������ˮ��Ӧ�� ���л�����һ���������� H2��ȫ�ӳ�,���������һ�ȴ����������
A��5�� B��6�� C��7�� D��8��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com