
����ͭ��һ����Ҫ�Ĺ�ҵ��Ʒ����ѧ���кܶ��������ȡ����ͭ���ٽ�ͭ���������С����Ƚ�ͭ��O2��Ӧ����CuO�������������С��۽�ͭ����N2O4����������(�ܼ������μӷ�Ӧ)��Һ����������ͭ��һ��������������ʶ���жϴ������ (����)��
A������������úͻ�����
B���������У����Ӿ��úͻ����ĽǶȿ��ǣ���ϡ�������Ũ�����
C����������Ҫ������Դ���Ի�����������Ⱦ
D����������N2O4�������������ǻ�ԭ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������2�������飻��2��2���������飻�����飻�ܱ��飻�ݶ��飻
�����ǵķе��ɸߵ��͵�˳��������ȷ����
A����>��>��>��>�� B����>��>��>��>��
C����>��>��>��>�� D����>��>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ輰�仯����������У�������� (����)��
A���������õİ뵼�����
B��������������ʯ��ʯ��Ӧ��SiO2��CaCO3 CaSiO3��CO2��
CaSiO3��CO2��
C�������ý�̿��ԭ�������������裺SiO2��2C Si��2CO��
Si��2CO��
D��ˮ�����Ҫ�ɷ���Na2SiO3��CaSiO3��SiO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ���ܱ�ʾ��ط�Ӧ�����������ʵ����ı仯����(�ᡢ�����굥λ��mol) (����)��
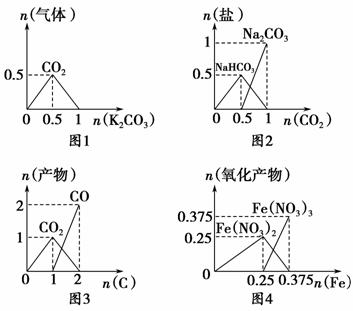
A��ͼ1��n(HCl)��1 mol��K2CO3���뵽HCl��Һ�У��ڳ������������ɵ�����
B��ͼ2��n(NaOH)��1 mol��CO2ͨ�뵽NaOH��Һ�з�Ӧ���ɵ���
C��ͼ3��n(O2)��1 mol��������C��O2���ܱ������е�������
D��ͼ4��n(HNO3)��1 mol��Fe��ϡHNO3��Ӧ���ɵ���������(��ԭ����ΪNO)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100 mL��NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ���CO2�����V(��״��)��M������(W)�Ĺ�ϵ��ͼ��ʾ��
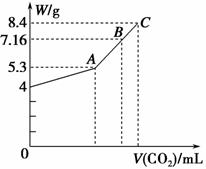
�Խ���������⣺
(1)A��ʱ����ɫ����M�Ļ�ѧʽΪ________��ͨ���״���µ�CO2�����Ϊ________ mL��
(2)C��ʱ����ɫ����M�Ļ�ѧʽΪ________��ͨ���״���µ�CO2�����Ϊ________ mL��
(3)B��ʱM����ɳɷ�Ϊ________(�û�ѧʽ��ʾ)��ͨ��ı�״���µ�CO2�����Ϊ________ mL��
(4)��NaOH��Һ�����ʵ���Ũ��Ϊ________��
(5)��μ���B����Һ�е������ӣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������FeCu�Ͻ���Ʒ(��������ΪFe2O3��CuO)��5.76 g�������´�����
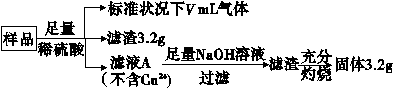
����˵����ȷ���� (����)��
A����ҺA�е�������ΪFe2����Fe3����H��
B����Ʒ��FeԪ�ص�����Ϊ2.24 g
C����Ʒ��CuO������Ϊ4.0 g
D��V��896 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��Բ�ཻ����A��B��C��D�ֱ��ʾ�����ʼ�ķ�Ӧ�����и���Ӧ��Ӧ�����ӷ���ʽ��д����ȷ���� (����)��
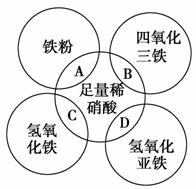
A��Fe��4H����NO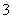 ===Fe3����NO����2H2O
===Fe3����NO����2H2O
B��Fe3O4��8H��===Fe2����2Fe3����4H2O
C��Fe(OH)3��3H��===Fe3����3H2O
D��3Fe(OH)2��10H����NO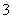 ===3Fe3����NO����8H2O
===3Fe3����NO����8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�п��ܺ��д�����Mg2����Al3����H����Cl��������OH���������Һ����μ���0.5 mol��L��1 NaOH��Һ�����ɳ����������ͼ���NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ������ж�ԭ��Һ�� (����)��
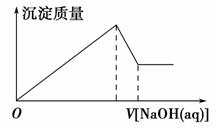
A����Mg2����û��Al3���� B����Al3����û��Mg2��
C����Mg2����Al3���� D�������H����Mg2����Al3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��̬������X����C��H��O����Ԫ�أ�����֪������������X��C��������������X��H��������������X�ڱ�״���µ��������X������������ܶȡ���X����������ȷ���û�����ķ���ʽ�����������������(����)
A���٢ڢ� B���ڢۢ� C���٢ۢ� D���٢�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com