
������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��
2FeSO4��7H2O ![]() Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ���ǣ� ��
Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ���ǣ� ��
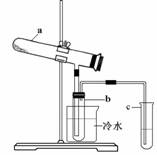
A��������Ӧ�����������ͨ��BaCl2��Һ�У������ij���ΪBaSO3��BaSO4
B��b�в�������ɫʯ����Һ���ɼ��������H+��SO42��
C��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ�ΪNaOH��Һ
D��b�����õ����������������Ϊ29.5%
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������������ϵķ��������̷�Ϊԭ�ϣ������������գ���Ӧ�Ļ�ѧ����ʽΪ��
������������ϵķ��������̷�Ϊԭ�ϣ������������գ���Ӧ�Ļ�ѧ����ʽΪ��
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18����40�����ǰ��������������ϵķ����ǣ����̷���FeSO4��7H2O��Ϊԭ�������������գ�Ȼ�������������£��õ�һ��Һ�壬��֮Ϊ���̷��͡���
��1�������������������к��еijɷ���________________________��
��2���̷��͵ijɷ���________________________��
��3���̷�������������________________________��
��4�����������������________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ҵ���Ի�����Ϊԭ������������Ҫ��Ϊ�����ν��У����ڷ���¯�����ջ�����SO2�Ĵ���������SO3�����ա���ش����м����й����Ṥҵ�еļ������⡣
��1��������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��2FeSO4��7H2O ![]() Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )
Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )

A��������Ӧ�����������ͨ��BaCl2��Һ�У������ij���ΪBaSO3��BaSO4
B��b�в�������ɫʯ����Һ���ɼ��������H+��SO42��
C��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ�ΪNaOH��Һ
D��b�����õ����������������Ϊ29.5%
(2)�ӷ���¯�г�����¯�����뾭������ϴ�ӡ���������Ӵ��ң�����ҪĿ����__________��
(3)�Ӵ������Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�������Һ�Ƚ���ʱͨ��ʹ�õ�������______________��
(4)�Ӵ�������Ҫ��Ӧ��SO2�Ĵ�����������������Ĺ����У�����ý(V2O5)�����ܼӿ���������������ٶȣ����˾������������⣬������Ϊ��Ӧ�����л�������һ�������м���(��ͼ)��c���Ļ�ѧ����ʽ�ɱ�ʾΪ_______________________��

(5) ��ҵ����������Ϊԭ����������������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ������Ṥҵ���ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ����β��ͨ���ĩ״��̼��ƻ���ʯ�ҵ�����Һ�У�����һϵ�д�����õ�һ����Է�������Ϊ172�Ļ���ԭ��J����д��J�Ļ�ѧʽ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18�֣������ǻ�ѧ��ҵ����Ҫ�IJ�Ʒ֮һ����ҵ�Ʒ����¡�
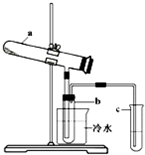
��1��������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��2FeSO4��7H2OFe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ�
���Թ�b�еõ�����Ҫ������ ������ò���ķ����ǣ�������ӷ���ʽ��Ҫ˵���� ��
��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ��� ��������Ӧ��ʵ�������� ��
�� �������������Ũ�ȣ����ʵ�����������Ϊ ��
��2��Ŀǰ���ҹ����á��Ӵ����������ᣬ�����豸��ͼ��ʾ��
��ͼ���豸A�������� ��a��b������������Ļ�ѧʽ�ֱ�Ϊ �� ��
���йؽӴ��������������˵���У�����ȷ���� ��
A�� ��������ĽӴ������ںϳ����з���
B���������õ�������Ũ��Ϊ98��
C�����պ���48���Ļ�����ʱ����FeS2��ʧ��2������S��ʧ4��
D��Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת����
��3�����Ż�ѧ��ҵ�ķ�չ�����Ӵ�����ȫ������ˡ��̷��ȷֽⷨ����������ۺ�Ч��ĽǶ�ָ�����Ӵ���������������ƣ��� �����ɱ��͡��� ԭ���á��� ���� ���� ���� ���ɲ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ������ǰ��Ӧѵ���������ۺϣ���ѧ���� ���ͣ�ʵ����
��18�֣������ǻ�ѧ��ҵ����Ҫ�IJ�Ʒ֮һ����ҵ�Ʒ����¡�
��1��������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��2FeSO4��7H2O Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ�
Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ�
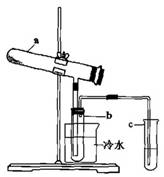
���Թ�b�еõ�����Ҫ������ ������ò���ķ����ǣ�������ӷ���ʽ��Ҫ˵���� ��
��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ��� ��������Ӧ��ʵ�������� ��
�� �������������Ũ�ȣ����ʵ�����������Ϊ ��
��2��Ŀǰ���ҹ����á��Ӵ����������ᣬ�����豸��ͼ��ʾ��
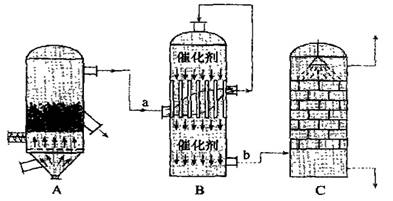
��ͼ���豸A�������� ��a��b������������Ļ�ѧʽ�ֱ�Ϊ �� ��
���йؽӴ��������������˵���У�����ȷ���� ��
A�� ��������ĽӴ������ںϳ����з���
B���������õ�������Ũ��Ϊ98��
C�����պ���48���Ļ�����ʱ����FeS2��ʧ��2������S��ʧ4��
D�� Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת����
��3�����Ż�ѧ��ҵ�ķ�չ�����Ӵ�����ȫ������ˡ��̷��ȷֽⷨ����������ۺ�Ч��ĽǶ�ָ�����Ӵ���������������ƣ��� �����ɱ��͡��� ԭ���á��� ���� ���� ���� ���ɲ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com