
A¡¢B¡¢C¡¢D¡¢E¡¢F¡¢G¡¢H¡¢I¡¢J¾ùΪÓĐ»ú»¯ºÏÎ¸ù¾ỬÔÏ¿̣ͼ£¬»Ø´đÎỀ⣺
(1)BºÍC¾ùΪÓĐÖ§Á´µÄÓĐ»ú»¯ºÏÎBµÄ½á¹¹¼̣ʽΪ__________£»CÔÚŨẠ́Ëá×÷ÓĂϼÓÈÈ·´Ó¦Ö»ÄÜÉú³É̉»ÖÖÏ©̀₫D£¬DµÄ½á¹¹¼̣ʽΪ__________________¡£
(2)GÄÜ·¢Éú̉ø¾µ·´Ó¦£¬̉²ÄÜʹäåµÄËÄÂÈ»¯̀¼ÈÜ̉ºÍÊÉ«£¬ỘGµÄ½á¹¹¼̣ʽΪ________¡£
(3)Đ´³ö¢ƯµÄ»¯Ñ§·´Ó¦·½³̀ʽ____________________________________________¡£¢àµÄ»¯Ñ§·´Ó¦·½³̀ʽ____________________________________________¡£
(4)¢ÙµÄ·´Ó¦ÀàĐÍ________£¬¢ÜµÄ·´Ó¦ÀàĐÍ________£¬¢ßµÄ·´Ó¦ÀàĐÍ________¡£
(5)ÓëH¾ßÓĐÏàͬ¹ÙÄÜÍŵÄHµÄËùÓĐͬ·Ö̉́¹¹̀åµÄ½á¹¹¼̣ʽΪ___________________¡£
£¨1£©¡¢CH3-CH(CH3)-COOH CH2=C(CH3)2
£¨2£©¡¢CH2=C(CH3)-CHO
£¨3£©¡¢CH2=C(CH3)-CH2Cl + NaOH ¡ª¡ªCH2=C(CH3)COOH + CH3OH ¡ª¡ª
£¨4£©¡¢Ë®½â·´Ó¦£¨È¡´ú·´Ó¦£© È¡´ú·´Ó¦ Ñơ»¯·´Ó¦
£¨5£©¡¢CH2=CH-CH2-COOH CH3-CH=CH-COOH
½âÎöÊỒâ·ÖÎö£º£¨1£©BΪôÈËáÇ̉º¬ÓĐÖ§Á´£¬¹ÊΪCH3-CH(CH3)-COOH£»CΪ´¼ÓĐ4Öֽṹ£¬º¬ÓĐÖ§Á´ÓĐÁ½ÖÖ£¬(CH3)3C-OHºÍ(CH3)2CH2-CH2OH£¬ÏûÈ¥²úÎï¾ùÖ»ÓĐ̉»ÖÖÇ̉Ïàͬ£»£¨2£©µÚ¢ÜÊÇÍé̀₫»ùµÄ±ËعâƠƠÈ¡´ú·´Ó¦£¬µÚ¢ƯΪˮ½â·´Ó¦Éú³É´¼£¬µÚ¢̃Ϊ´¼µÄ´ß»¯Ñơ»¯Éú³ÉÈ©£¬º¬ÓĐ̀¼̀¼Ë«¼ü£¬¹ÊGΪCH2=C(CH3)-CHO£»£¨3£©µÚ¢ßÈ©Ñơ»¯Éú³ÉôÈËᣬµÚ¢àôÈËáÓë¼×´¼·¢Éúơ¥»¯·´Ó¦Éú³Éơ¥£»£¨4£©Î»ÖẲ́¹¹¡£
¿¼µă£º¿¼²éÓĐ»úºÏ³ÉÖĐÎïÖÊת±äÏà¹ØµÄ²úÎï¡¢·´Ó¦ÀàĐÍ¡¢·½³̀ʽÊéĐ´µÈÓĐ¹ØÎỀâ¡£



| Ä꼶 | ¸ßÖĐ¿Î³̀ | Ä꼶 | ³ơÖĐ¿Î³̀ |
| ¸ß̉» | ¸ß̉»Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ở» | ³ở»Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ß¶₫ | ¸ß¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ơ¶₫ | ³ơ¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ßÈư | ¸ßÈưĂâ·Ñ¿Î³̀ÍƼö£¡ | ³ơÈư | ³ơÈưĂâ·Ñ¿Î³̀ÍƼö£¡ |
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
¸ù¾ỬªÇó̀î¿Ơ£º
£¨1£©Ö»ÓĂ¼üÏßÀ´±íʾ̀¼¼Ü£¬Á½¸ùµ¥¼üÖ®¼ä»̣̉»¸ùË«¼üºÍ̉»¸ùµ¥¼üÖ®¼äµÄ¼Đ½ÇΪ120?£¬̉»¸ùµ¥¼üºÍ̉»¸ùÈư¼üÖ®¼äµÄ¼Đ½ÇΪ180?£¬¶ø·Ö×ÓÖеÄ̀¼Çâ¼ü¡¢̀¼Ô×Ó¼°Óë̀¼Ô×ÓÏàÁ¬µÄÇâÔ×Ó¾ùÊ¡ÂÔ£¬¶øÆäËûÔÓÔ×Ó¼°ÓëÔÓÔ×ÓÏàÁ¬µÄÇâÔ×ÓĐë±£Áô¡£Ă¿¸ö¶ËµăºÍ¹Ơ½Ç´¦¶¼´ú±í̉»¸ö̀¼¡£ÓĂƠâÖÖ·½Ê½±íʾµÄ½á¹¹Ê½Îª¼üÏßʽ¡£Đ´³öÏÂÁĐÓĐ»úÎïµÄ¼üÏßʽ£º
¢ÙCH3£¨CH2£©2COOH____________________£»¢Ú £º__________________¡£
£º__________________¡£
£¨2£©Đ´³öÏÂÁĐÔ×ÓÍŵĵç×Óʽ£º¢Ù¼×»ù _______________ £»¢ÚÇâÑơ¸ùÀë×Ó _____________
£¨3£©Đ´³öÓĐ»úÎï µÄĂû³Æ _______________________________________
µÄĂû³Æ _______________________________________
£¨4£©ÔÚÓĐ»úÎï·Ö×ÓÖĐÈôij̉»¸ö̀¼Ô×ÓÁ¬½Ó4¸ö²»Í¬µÄÔ×Ó»̣»ùÍÅ£¬ỘƠâÖÖ̀¼Ô×Ó³ÆΪ¡°ÊÖĐỒ¼Ô×Ó¡±£®C7H16µÄͬ·Ö̉́¹¹̀åÖĐ¾ßÓĐ¡°ÊÖĐỒ¼Ô×Ó¡±µÄÓĐ ___________ÖÖ£®
£¨5£©Ä³ÓĐ»ú¸ß·Ö×Ó»¯ºÏÎïµÄ½á¹¹Æ¬¶ÎÈçÏ£º
ỘºÏ³ÉËüµÄµ¥̀åÊÇ ____________________________________¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
½á¹¹µÄÑĐ¾¿ÊÇÓĐ»ú»¯Ñ§×îÖØ̉ªµÄÑĐ¾¿Á́Ọ́£¬Ä³ÓĐ»úÎïX£¨C12H13O6Br£©·Ö×ÓÖĐº¬ÓжàÖÖ¹ÙÄÜÍÅ£¬Æä½á¹¹¼̣ʽÈçÏ£º£¨ÆäÖĐ¢ñ¡¢¢̣Ϊδ֪²¿·ÖµÄ½á¹¹£©¡£
ΪÍƲâXµÄ·Ö×ӽṹ£¬½øĐĐÈçÏÂͼת»¯£º

̉ÑÖªỊ̈DµÄË®ÈÜ̉ºÖеÎÈëFeCl3ÈÜ̉ºÏÔ×ÏÉ«£¬¶ÔDµÄ½á¹¹½øĐĐ¹âÆ×·ÖÎö£¬ÔÚÇâºË´Å¹²ƠñÆ×ÉÏÏÔʾֻÓĐÁ½ÖÖĐźš£M¡¢N»¥ÎªÍ¬·Ö̉́¹¹̀壬MÖĐº¬ÓĐ̉»¸öÁùÔ×Ó»·£¬NÄÜʹäåµÄËÄÂÈ»¯̀¼ÈÜ̉ºÍÊÉ«£¬GÄÜÓëNaHCO3ÈÜ̉º·´Ó¦¡£Çë»Ø´đ£º
£¨1£©D²»¿É̉Ô·¢ÉúµÄ·´Ó¦ÓĐ£¨Ñ¡̀îĐ̣ºÅ£©_______ ______£»
¢Ù¼Ó³É·´Ó¦ ¢ÚÏûÈ¥·´Ó¦ ¢ÛÑơ»¯·´Ó¦ ¢ÜÈ¡´ú·´Ó¦
£¨2£©Đ´³öÉÏͼת»¯ÖĐÉú³É MµÄ»¯Ñ§·½³̀ʽ:________________ _________________________________
£¨3£©̉ÑÖªỊ̈XÖĐ¼ÓÈëFeCl3ÈÜ̉º£¬ÄÜ·¢ÉúÏÔÉ«·´Ó¦£¬Đ´³öXµÄ̉»Öֽṹ¼̣ʽ:__ £»1mol¸Ă XÓë×ăÁ¿µÄNaOHÈÜ̉º×÷ÓĂ£¬×î¶à¿ÉÏûºÄNaOH_____ ______mol¡£
£¨4£©ÓĐ̉»ÖÖ»¯¹¤²úÆ·µÄÖĐ¼ä̀åWÓëG»¥ÎªÍ¬·Ö̉́¹¹̀壬WµÄ·Ö×ÓÖĐÖ»º¬ÓĐôÈ»ù¡¢ôÇ»ùºÍÈ©»ùÈưÖÖ¹ÙÄÜÍÅ£¬Ç̉ͬ̉»¸ö̀¼Ô×ÓÉϲ»ÄÜͬʱÁ¬ÓĐÁ½¸öôÇ»ù¡£Đ´³öWµÄ¿ÉÄܽṹ¼̣ʽ_____ ___¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
°¢Ë¹Æ¥ÁÖ£¨aspirin£©µÄÓĐЧ³É·ÖÊÇ̉̉ơ£Ë®ÑîËᣬËüÊÇ19ÊÀ¼ÍÄ©ºÏ³É³É¹¦µÄ£¬×÷Ϊ̉»¸öÓĐЧµÄ½âÈÈֹʹ¡¢ÖÎÁƸĐĂ°µÄ̉©ÎÖÁ½ñÈÔ¹ă·ºÊ¹ÓĂ£¬ÓĐ¹Ø±¨µÀ±íĂ÷£¬ÈËĂÇƠưÔÚ·¢ÏÖËüµÄÄ³Đ©Đ¹¦ÄÜ¡£°¢Ë¹Æ¥ÁÖÊÇÓÉË®ÑîËᣨÁÚôÇ»ù±½¼×ËᣩÓë̉̉Ëáôû½øĐĐơ¥»¯·´Ó¦¶øµĂµÄ¡£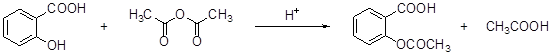
Ë®ÑîËá ̉̉Ëáôû ̉̉ơ£Ë®ÑîËá
£¨1£©̉̉ơ£Ë®ÑîËáµÄº¬Ñơ¹ÙÄÜÍÅ·Ö±đÊÇ ºÍơ¥»ù¡£
£¨2£©̉̉ơ£Ë®ÑîËá²»Ó¦¾ßÓеÄĐÔÖÊ£¨ £©
| A£®ÓëNaOHÈÜ̉º·´Ó¦ | B£®Óë½đÊôÄÆ·´Ó¦ |
| C£®Óë̉̉Ëá·¢Éúơ¥»¯·´Ó¦ | D£®Óë̉̉´¼·¢Éúơ¥»¯·´Ó¦ |
| ´ÎÊư Êư¾Ư | 1 | 2 | 3 |
| ³ơʼ¶ÁÊư | 2£®00 | 12£®50 | 5£®20 |
| ×îºó¶ÁÊư | 27£®10 | 37£®40 | 33£®20 |
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
(10·Ö)ÓĐ»úÎïA¡¢B¡¢C¡¢D¡¢E¡¢FÓĐ̉»¸öÏàͬ¹ÙÄÜÍÅ£¬ËüĂÇÖ®¼äÓĐÏÂͼת»¯¹Øϵ£º
̉ÑÖª£º
¢Ù RCH2OH RCHO
RCHO
¢Ú
»Ø´đÏÂÁĐÎỀ⣺
£¨1£©Đ´³öD¡¢FµÄ½á¹¹¼̣ʽ£ºD £¬F
£¨2£©BµÄĂû³ÆÊÇ £¬ÊµÏÖC¡úEת»¯µÄ·´Ó¦̀ơ¼₫ÊÇ £»
£¨3£©Đ´³öAÓë̉ø°±ÈÜ̉º·´Ó¦µÄ»¯Ñ§·½³̀ʽ£º £»
£¨4£©Đ´³öͬʱÂú×ăÏÂÁĐÈư¸ö̀ơ¼₫µÄEµÄËùÓĐͬ·Ö̉́¹¹̀å £¨²»°üÀ¨Á¢̀å̉́¹¹£©¡£
a£®±½»·ÉÏÖ»ÓĐ̉»¸ö²àÁ´ b£®Äܹ»·¢ÉúË®½â·´Ó¦ c£®º¬̉»¸ö̀¼̀¼Ë«¼ü
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
£¨1£©ÓĐÏÂÁĐÁù×éÎïÖÊ£ºÊôÓÚͬϵÎïµÄÊÇ £¬ÊôÓÚͬ·Ö̉́¹¹̀åÊÇ £¬ÊôÓÚͬÖÖÎïÖʵÄÊÇ ¡££῭îĐ̣ºÅ£© 
£¨2£©A£®Ä³̀₫Óë2±¶µÄÇâÆø¼Ó³ÉºóµĂµ½2£¬2-¶₫¼×»ù¶¡Í飬°´ÏµÍ³ĂüĂû·¨,¸Ằ₫µÄĂû³ÆÊÇ ¡£
B£®ÏÂͼÊÇijÓĐ»úÎï½á¹¹¼̣ʽ, °´ÏµÍ³ĂüĂû·¨,¸ĂÓĐ»úÎïµÄĂüĂûƠưÈ·µÄÊÇ ¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
½đ¸ƠŸ†°·ºÍ´ï·Æ¶¼ÊÇ·ÀÖÎÈË´«È¾ÇƯÁ÷¸ĐµÄ³£ÓĐ̉©¡£½đ¸ƠÍé°·½á¹¹Ê½£º
£¨1£©½đ¸ƠÍé°·µÄ·Ö×ÓʽΪ_______¡£
£¨2£©½đ¸ƠÍé°·µÄͬ·Ö̉́¹¹̀åÖĐ____£῭î¡°´æÔÚ¡±»̣¡°²»´æÔÚ¡±£©·¼Ïă×廯ºÏÎï¡£
Éú²ú´ï·ÆµÄÖ÷̉ªÔÁÏÊÇç²ƯËá£¬Ă§²ƯËá´æÔÚ̉ÔÏÂת»¯¹Øϵ£º
Aº¬±½»·£¬Æä±½»·ÉÏÈôÓĐ̉»¸öÇâÔ×Ó±»ÂÈÔ×ÓÈ¡´ú£¬½«¿ÉÄܲúÉúÁ½ÖÖͬ·Ö̉́¹¹̀å¡£
£¨3£©Ă§²ƯËá¡úAµÄ»¯Ñ§·½³̀ʽ£º_______£¬¸Ă·´Ó¦ÀàĐÍΪ_______¡£
£¨4£©BµÄ½á¹¹¼̣ʽΪ_______¡£
£¨5£©AµÄ̉»ÖÖͬ·Ö̉́¹¹̀åË®ÑîËᣨ £©̉²ÊÇ̉½̉©¹¤̉µµÄÖØ̉ªÔÁÏ£¬ÏÂÁĐÓĐ»úÎïÊôÓÚË®ÑîËáͬϵÎïµÄÊÇ_______£῭îÑ¡Ïî±àºÅ£©¡£
£©̉²ÊÇ̉½̉©¹¤̉µµÄÖØ̉ªÔÁÏ£¬ÏÂÁĐÓĐ»úÎïÊôÓÚË®ÑîËáͬϵÎïµÄÊÇ_______£῭îÑ¡Ïî±àºÅ£©¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
·¼Ïăơ¥£¨G£©ÊÇ̉»ÖÖÏă¾«µÄµ÷Ïă¼Á£¬·Ö×ÓʽΪC11H12O2£¬¿ÉÓÉÏÂÁĐ·Ïߺϳɣº
Çë»Ø´đÏÂÁĐÎỀ⣺
£¨1£©BÖĐº¬ÓĐµÄ¹ÙÄÜÍŵÄĂû³ÆÊÇ ¡£
£¨2£©¢Û¡¢¢ÜµÄ·´Ó¦ÀàĐÍ·Ö±đÊÇ ¡¢ ¡£
£¨3£©AµÄĂû³ÆÊÇ £῭îϵͳĂüĂû£©¡£
£¨4£©BµÄͬ·Ö̉́¹¹̀åÓжàÖÖ£¬ÆäÖĐ̉»ÖÖÄÜ·¢ÉúË®½â·´Ó¦£¬Ç̉ºË´Å¹²ƠñÇâÆ×ÏÔʾÓĐËĸöÎüÊƠ·å¡£Đ´³ö¸Ăͬ·Ö̉́¹¹̀åµÄ½á¹¹¼̣ʽ£º ¡£
£¨5£©·´Ó¦¢̃µÄ»¯Ñ§·½³̀ʽÊÇ ¡£
£¨6£©̉ÔDÓë¼×´¼ơ¥»¯·´Ó¦ºóµÄÓĐ»ú²úÎïΪµ¥̀壬ÔÚ´ß»¯¼Á×÷ÓĂϾۺϳÉ̉»Öָ߷Ö×Ó»¯ºÏÎË׳ÆÓĐ»ú²£Á§£©£¬Đ´³öºÏ³ÉÓĐ»ú²£Á§µÄ»¯Ñ§·½³̀ʽ£º ¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º̀î¿Ờâ
6-ôÊ»ù¸ưËáÊÇ̉»ÖÖÖØ̉ªµÄ»¯¹¤ÖĐ¼ä̀壬ÏÂĂæºÏ³ÉËüµÄÁ÷³̀ͼ£º
̉ÑÖª£º 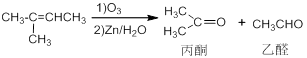
£¨1£©·´Ó¦¢ÙµÄ̀ơ¼₫ÊÇ £¬·´Ó¦ÀàĐÍÊÇ ¡£
£¨2£©ÏÂÁĐ˵·¨ÖĐƠưÈ·µÄÊÇ £º
a£®1molCÓë×ăÁ¿µÄNa·´Ó¦Éú³É1molH2 b£®CÄܱ»´ß»¯Ñơ»¯³Éͪ
c£®Ni´ß»¯ÏÂ1molG×î¶àÖ»ÄÜÓë1molH2¼Ó³É d£®FÄÜ·¢ÉúÏûÈ¥·´Ó¦Éú³ÉÁ½ÖÖ²»Í¬Ï©̀₫
£¨3£©EÓëĐÂÖÆCu(OH)2·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ ¡£
£¨4£©GµÄͬ·Ö̉́¹¹̀åÓжàÖÖ¡£ÇëĐ´³ö½á¹¹ÖĐº¬ÓĐ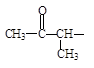 ¡¢Ç̉ÊôÓÚơ¥ÀàµÄͬ·Ö̉́¹¹
¡¢Ç̉ÊôÓÚơ¥ÀàµÄͬ·Ö̉́¹¹
̀壺 ¡¢ ¡¢ ¡£
£¨5£©̉ÑÖª¡°Diels-Alder·´Ó¦¡±Îª£º ¡££¬
¡££¬
ÎïÖÊDÓë߻ૣ¨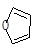 £©̉²¿É̉Ô·¢Éú¡°Diels-Alder·´Ó¦¡±£¬¸Ă»¯Ñ§·´Ó¦·½³̀ʽΪ£º
£©̉²¿É̉Ô·¢Éú¡°Diels-Alder·´Ó¦¡±£¬¸Ă»¯Ñ§·´Ó¦·½³̀ʽΪ£º
¡£
²é¿´´đ°¸ºÍ½âÎö>>
°Ù¶ÈÖÂĐÅ - Á·Ï°²áÁбí - ÊỒâÁбí
º₫±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨Æ½̀¨ | ÍøÉÏÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | µçĐÅƠ©Æ¾Ù±¨×¨Çø | ÉæÀúÊ·ĐéÎ̃Ö÷̉åÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com