
£Ø18·Ö£©æĘѧĢ½¾æ½į¹ūµÄ×¼Č·ŠŌĄ“×ŌÓŚĢ½¾æ»ī¶ÆÖŠŃŠ¾æ·½·ØµÄæĘѧŠŌ£¬ŃŠ¾æ¹ż³ĢµÄ¼Ę»®ŠŌ”¢ŃŠ¾æÄæµÄĆ÷Č·ŠŌ”£
£ØŅ»£©Ń§Éś£Ø¼×£©Ó¦ÓĆĻĀĶ¼×°ÖĆ£ØA£©ĖłŹ¾ŅāµÄ·½·ØŃŠ¾æĀČĘųµÄŠŌÖŹ£¬ĘäÖŠĘųĢåµÄÖ÷ŅŖ³É·ÖŹĒĀČĘų£Øŗ¬ÓŠæÕĘųŗĶĖ®ÕōĘų£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆĻīŃŠ¾æ(ŹµŃé)µÄÖ÷ŅŖÄæµÄŹĒ
£Ø2£©ÅØĮņĖįµÄ×÷ÓĆŹĒ ”£ÓėŃŠ¾æÄæµÄÖ±½ÓĻą¹ŲµÄŹµŃéĻÖĻóŹĒ ________________ _ ”£
£Ø3£©ŠéæņÖŠµÄ×°ÖĆӦєŌń £ØĢī”°B”±»ņ”°C”±£©£¬ĘäŹ¢·ÅµÄŹŌ¼Į
ĪŖ £ØĢīŹŌ¼ĮĆū³Ę£© £»Ź¹ÓĆøĆ×°ÖƵÄÄæµÄŹĒ £»øĆ×°ÖĆÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø¶ž£©Ń§Éś£ØŅŅ£©Éč¼ĘŹµŃéĢ½¾æ½šŹōĀĮ±ķĆęŃõ»ÆĤµÄŠŌÖŹ£ŗ½«ĀĮʬ£Øŗ¬Ńõ»ÆĤ£©Ķ¶ČėÅØĀČ»ÆĶČÜŅŗÖŠ£¬ĀĮ±ķĆęŗÜæģ³öĻÖŅ»²ćŗ£Ćą×“°µŗģÉ«ĪļÖŹ”£ČōÓĆĶ¬ŃłµÄĀĮʬĶ¶ČėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗÖŠ£¬ŌŚ¶ĢŹ±¼äÄŚĀĮʬĪŽĆ÷ĻŌ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĀĮʬ±ķĆę³öĻֵݵŗģÉ«ĪļÖŹµÄ¹ż³ĢÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ŹĒ ”£
£Ø2£©Ķ¬Ń§£ØŅŅ£©ČĻĪŖ£ŗĀĮÓėĀČ»ÆĶČÜŅŗÄÜŃøĖŁ·“Ó¦£¬¶ųÓėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗŌŚ¶ĢŹ±¼äÄŚ²»·“Ó¦µÄŌŅņŹĒ”°ĀČĄė×ÓÄÜĘĘ»µŃõ»ÆĀĮ±ķĆ걔Ĥ,¶ųĮņĖįøłĄė×Ó²»ÄÜ”±”£²¢Éč¼ĘČēĻĀŹµŃé·½°ø½ųŠŠŃéÖ¤£¬ĒėÄćøł¾ŻĘäĖ¼Ā·Ķź³ÉĻĀĮŠæÕøń£ŗ
[ŹµŃé·½°ø] ŌŚĮņĖįĶČÜŅŗÖŠ¼ÓČėĀĮʬ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ŌŁ¼ÓČė £ØĢīŹŌ¼ĮĆū³Ę£©£¬Čō·“Ó¦Ć÷ĻŌ¼ÓæģĮĖ£¬ĖµĆ÷ÉĻŹöĶʶĻÕżČ·”£
£Ø18·Ö£©£ØŅ»£©
£Ø1£©±Č½ĻøÉŌļµÄĀČĘųŗĶ³±ŹŖµÄĀČĘųµÄĘư׊Ō£Ø»ņŃŠ¾æĀČĘųµÄĘư׊ŌŹµŃéµČŗĻĄķ“š°ø¾łæÉ£©£Ø2·Ö£©
£Ø2£©ĪüŹÕĘųĢåÖŠµÄĖ®£» øÉŌļµÄÓŠÉ«²¼Ģõ²»ĶŹÉ«£¬ŹŖČóµÄÓŠÉ«²¼ĢõĶŹÉ«£ØŗĻĄķ“š°ø¾łæÉ£©
£Øø÷æÕ2·Ö£¬¹²4·Ö£©
£Ø3£©B £»NaOHČÜŅŗ£»·ĄÖ¹ÓŠ¶¾µÄCl2ĪŪČ¾æÕĘų£»2NaOH + Cl2== NaCl + NaClO+ H2O £Øø÷æÕ2·Ö£¬¹²8·Ö£©
£Ø¶ž£©£Ø1£©2Al+3Cu2+£½3Cu+2Al3+;£Ø2·Ö£©
£Ø2£©ĀČ»ÆÄĘ£Ø¼ÓČėŃĪĖį“ķĪ󣩣Ø2·Ö£©
½āĪö:ĀŌ


 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğø£½ØŹ”Ź¦“óø½ÖŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø18·Ö£©æĘѧĢ½¾æ½į¹ūµÄ×¼Č·ŠŌĄ“×ŌÓŚĢ½¾æ»ī¶ÆÖŠŃŠ¾æ·½·ØµÄæĘѧŠŌ£¬ŃŠ¾æ¹ż³ĢµÄ¼Ę»®ŠŌ”¢ŃŠ¾æÄæµÄĆ÷Č·ŠŌ”£
£ØŅ»£©Ń§Éś£Ø¼×£©Ó¦ÓĆĻĀĶ¼×°ÖĆ£ØA£©ĖłŹ¾ŅāµÄ·½·ØŃŠ¾æĀČĘųµÄŠŌÖŹ£¬ĘäÖŠĘųĢåµÄÖ÷ŅŖ³É·ÖŹĒĀČĘų£Øŗ¬ÓŠæÕĘųŗĶĖ®ÕōĘų£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ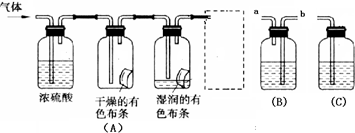
£Ø1£©øĆĻīŃŠ¾æ(ŹµŃé)µÄÖ÷ŅŖÄæµÄŹĒ
£Ø2£©ÅØĮņĖįµÄ×÷ÓĆŹĒ ”£ÓėŃŠ¾æÄæµÄÖ±½ÓĻą¹ŲµÄŹµŃéĻÖĻóŹĒ ________________ _ ”£
£Ø3£©ŠéæņÖŠµÄ×°ÖĆӦєŌń £ØĢī”°B”±»ņ”°C”±£©£¬ĘäŹ¢·ÅµÄŹŌ¼Į
ĪŖ £ØĢīŹŌ¼ĮĆū³Ę£©£»Ź¹ÓĆøĆ×°ÖƵÄÄæµÄŹĒ £»øĆ×°ÖĆÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø¶ž£©Ń§Éś£ØŅŅ£©Éč¼ĘŹµŃéĢ½¾æ½šŹōĀĮ±ķĆęŃõ»ÆĤµÄŠŌÖŹ£ŗ½«ĀĮʬ£Øŗ¬Ńõ»ÆĤ£©Ķ¶ČėÅØĀČ»ÆĶČÜŅŗÖŠ£¬ĀĮ±ķĆęŗÜæģ³öĻÖŅ»²ćŗ£Ćą×“°µŗģÉ«ĪļÖŹ”£ČōÓĆĶ¬ŃłµÄĀĮʬĶ¶ČėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗÖŠ£¬ŌŚ¶ĢŹ±¼äÄŚĀĮʬĪŽĆ÷ĻŌ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĀĮʬ±ķĆę³öĻֵݵŗģÉ«ĪļÖŹµÄ¹ż³ĢÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ŹĒ ”£
£Ø2£©Ķ¬Ń§£ØŅŅ£©ČĻĪŖ£ŗĀĮÓėĀČ»ÆĶČÜŅŗÄÜŃøĖŁ·“Ó¦£¬¶ųÓėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗŌŚ¶ĢŹ±¼äÄŚ²»·“Ó¦µÄŌŅņŹĒ”°ĀČĄė×ÓÄÜĘĘ»µŃõ»ÆĀĮ±ķĆ걔Ĥ,¶ųĮņĖįøłĄė×Ó²»ÄÜ”±”£²¢Éč¼ĘČēĻĀŹµŃé·½°ø½ųŠŠŃéÖ¤£¬ĒėÄćøł¾ŻĘäĖ¼Ā·Ķź³ÉĻĀĮŠæÕøń£ŗ
[ŹµŃé·½°ø] ŌŚĮņĖįĶČÜŅŗÖŠ¼ÓČėĀĮʬ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ŌŁ¼ÓČė £ØĢīŹŌ¼ĮĆū³Ę£©£¬Čō·“Ó¦Ć÷ĻŌ¼ÓæģĮĖ£¬ĖµĆ÷ÉĻŹöĶʶĻÕżČ·”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģø£½ØŹ”øßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø18·Ö£©æĘѧĢ½¾æ½į¹ūµÄ×¼Č·ŠŌĄ“×ŌÓŚĢ½¾æ»ī¶ÆÖŠŃŠ¾æ·½·ØµÄæĘѧŠŌ£¬ŃŠ¾æ¹ż³ĢµÄ¼Ę»®ŠŌ”¢ŃŠ¾æÄæµÄĆ÷Č·ŠŌ”£
£ØŅ»£©Ń§Éś£Ø¼×£©Ó¦ÓĆĻĀĶ¼×°ÖĆ£ØA£©ĖłŹ¾ŅāµÄ·½·ØŃŠ¾æĀČĘųµÄŠŌÖŹ£¬ĘäÖŠĘųĢåµÄÖ÷ŅŖ³É·ÖŹĒĀČĘų£Øŗ¬ÓŠæÕĘųŗĶĖ®ÕōĘų£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©øĆĻīŃŠ¾æ(ŹµŃé)µÄÖ÷ŅŖÄæµÄŹĒ
£Ø2£©ÅØĮņĖįµÄ×÷ÓĆŹĒ ”£ÓėŃŠ¾æÄæµÄÖ±½ÓĻą¹ŲµÄŹµŃéĻÖĻóŹĒ ________________ _ ”£
£Ø3£©ŠéæņÖŠµÄ×°ÖĆӦєŌń £ØĢī”°B”±»ņ”°C”±£©£¬ĘäŹ¢·ÅµÄŹŌ¼Į
ĪŖ £ØĢīŹŌ¼ĮĆū³Ę£© £»Ź¹ÓĆøĆ×°ÖƵÄÄæµÄŹĒ £»øĆ×°ÖĆÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø¶ž£©Ń§Éś£ØŅŅ£©Éč¼ĘŹµŃéĢ½¾æ½šŹōĀĮ±ķĆęŃõ»ÆĤµÄŠŌÖŹ£ŗ½«ĀĮʬ£Øŗ¬Ńõ»ÆĤ£©Ķ¶ČėÅØĀČ»ÆĶČÜŅŗÖŠ£¬ĀĮ±ķĆęŗÜæģ³öĻÖŅ»²ćŗ£Ćą×“°µŗģÉ«ĪļÖŹ”£ČōÓĆĶ¬ŃłµÄĀĮʬĶ¶ČėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗÖŠ£¬ŌŚ¶ĢŹ±¼äÄŚĀĮʬĪŽĆ÷ĻŌ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĀĮʬ±ķĆę³öĻֵݵŗģÉ«ĪļÖŹµÄ¹ż³ĢÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ŹĒ ”£
£Ø2£©Ķ¬Ń§£ØŅŅ£©ČĻĪŖ£ŗĀĮÓėĀČ»ÆĶČÜŅŗÄÜŃøĖŁ·“Ó¦£¬¶ųÓėĶ¬ÅØ¶ČµÄĮņĖįĶČÜŅŗŌŚ¶ĢŹ±¼äÄŚ²»·“Ó¦µÄŌŅņŹĒ”°ĀČĄė×ÓÄÜĘĘ»µŃõ»ÆĀĮ±ķĆ걔Ĥ,¶ųĮņĖįøłĄė×Ó²»ÄÜ”±”£²¢Éč¼ĘČēĻĀŹµŃé·½°ø½ųŠŠŃéÖ¤£¬ĒėÄćøł¾ŻĘäĖ¼Ā·Ķź³ÉĻĀĮŠæÕøń£ŗ
[ŹµŃé·½°ø] ŌŚĮņĖįĶČÜŅŗÖŠ¼ÓČėĀĮʬ£¬ĪŽĆ÷ĻŌĻÖĻó£¬ŌŁ¼ÓČė £ØĢīŹŌ¼ĮĆū³Ę£©£¬Čō·“Ó¦Ć÷ĻŌ¼ÓæģĮĖ£¬ĖµĆ÷ÉĻŹöĶʶĻÕżČ·”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com