
�������зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1����������պ���ʱ������Ҫ���Ǽ��⣬����Ҫ�õ���������
��������������ѡ��������������ñ����ĸ��д�ڿհ״���
A���ձ� B������ C�������� D�������� E���ƾ��� F��������
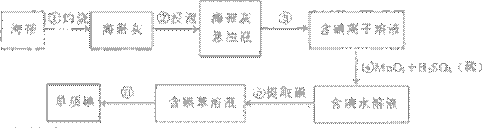
��2������۵�ʵ����������� �������Ŀ���ǴӺ��ⱽ��Һ�з������ͻ��ձ����ò����ʵ����������� ��
��3��������У�ijѧ��ѡ���ñ�����ȡ���������
��
��4�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ���
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | 2- 3 |
| O | 2- 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
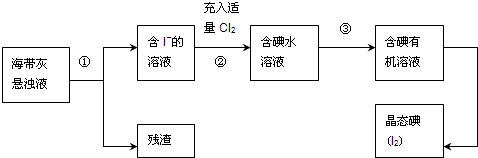
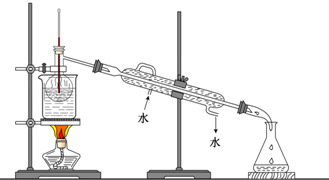
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| b |
| a(1-m) |
| 100b |
| a(1-m%) |
| b |
| a(1-m) |
| 100b |
| a(1-m%) |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
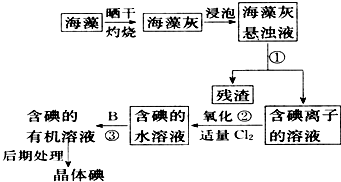 ����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ�������ӵ���ʽ���ڣ�ʵ���дӺ�����ȡ���������ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com