
·ÖĪö £Ø1£©¹¤Ņµ·ĻĘųÖŠSO2æɱ»ŹÆ»ŅĖ®ĪüŹÕ£¬Éś³É·ĻŌüĪŖCaSO3£¬ĘųĢåaŹĒ²»Äܱ»¹żĮæŹÆ»ŅĖ®ĪüŹÕµÄN2”¢NO”¢CO£¬ĘųĢåaÖŠĶØČėæÕĘų£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗ“¦Ąķŗóµ½µÄNaNO2£¬ĶØČėæÕĘųµ«²»ÄܹżĮ棬·ńŌņµĆµ½ĻõĖįÄĘ£¬NaNO2Óėŗ¬ÓŠNH4+µÄČÜŅŗ·“Ӧɜ³ÉĪŽĪŪČ¾ĘųĢå£¬Ó¦Éś³ÉµŖĘų£¬ŌņĘųĢåbŗ¬ÓŠCO”¢N2£¬²¶»ń¼ĮĖł²¶»ńµÄĘųĢåÖ÷ŅŖŹĒCO£»
£Ø2£©Ä³ĪŽÉ«¹¤Ņµ·ĻĖ®ÖŠæÉÄÜŗ¬ÓŠNH4+”¢Na+”¢Al3+”¢Cu2+”¢Cl-”¢SO42-”¢CO32-µČĄė×ÓÖŠµÄ¼øÖÖĄė×Ó£¬ĖµĆ÷Ņ»¶Ø²»ŗ¬Cu2+£¬
a£®Č”10mLøĆČÜŅŗÓŚŹŌ¹ÜÖŠµĪ¼ÓBa£ØNO3£©2ČÜŅŗ£¬¼ÓĻ”ĻõĖįĖį»Æŗó¹żĀĖµĆµ½0.04mol°×É«³Įµķ£¬Éś³É³ĮµķĪŖBaSO4£¬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠSO42-£¬ĻņĀĖŅŗÖŠ¼ÓČėAgNO3ČÜŅŗĪ“¼ū³Įµķ²śÉś£¬ŌņČÜŅŗ֊ƻӊCl-£»
b£®ĮķČ”10mLøĆČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼ÓNaOHČÜŅŗ²śÉś°×É«³Įµķ£¬µ±³ĮµķŌö¼Óµ½Ņ»¶ØĮæŗóæŖŹ¼²śÉśĘųĢ壬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠNH4+£¬×īŗó³ĮµķĶźČ«Čܽā£®ŌņĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠAl3+£¬Ņ»¶Ø²»ŗ¬Ģ¼ĖįøłĄė×Ó£»
¢ŁŅĄ¾ŻÉĻŹö·ÖĪöæÉÖŖ£¬øĆ·ĻĖ®ÖŠŅ»¶Øŗ¬ÓŠµÄĄė×ÓÓŠ£ŗNH4+”¢Al3+”¢SO42-£¬Ņ»¶Ø²»ŗ¬Ģ¼ĖįøłĄė×Ó£¬½įŗĻµēŗÉŹŲŗć·ÖĪöÅŠ¶ĻŹĒ·ńŗ¬ÓŠÄĘĄė×Ó£»
¢Śøł¾ŻČÜŅŗÅäÖĘÖŖŹ¶½ų·ÖĪöÅäÖĘŹ±¾ßĢå²½ÖčŗĶŹ¹ÓƵÄŅĒĘ÷½ųŠŠ½ā“š£»
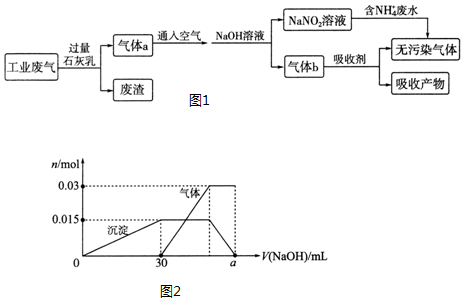
¢ŪĮķČ”10mLøĆČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼ÓNaOHČÜŅŗ²śÉś°×É«³Įµķ£¬ŌņĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠAl3+£¬Al3++3OH-=Al£ØOH£©3”ż£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµ£¬ÖŖČÜŅŗÖŠŗ¬ÓŠAl3+ĪŖ0.015molŠčOH-ĪŖ0.045mol£¬ĒāŃõ»ÆÄĘČÜŅŗĢå»ż=30ml£¬ÅضČc=$\frac{0.045mol}{0.030L}$=1.5mol/L£¬µ±³ĮµķŌö¼Óµ½Ņ»¶ØĮæŗóæŖŹ¼²śÉśĘųĢ壬øł¾ŻNH4++OH-=NH3”ü+H2O½įŗĻĶ¼Ļó£¬ÖŖČÜŅŗÖŠŗ¬ÓŠNH4+ĪŖ0.03molŠčOH-ĪŖ0.03mol£»×īŗó³ĮµķĶźČ«ČܽāŹĒÓÉÓŚĒāŃõ»ÆĀĮÓėNaOH¼ĢŠų·“Ó¦£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3+OH-=AlO2-+2H2O£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµ£¬ŠčOH-ĪŖ0.015mol£¬¾Ż“Ė¼ĘĖća£®
½ā“š ½ā£ŗ£Ø1£©¹¤Ņµ·ĻĘųÖŠSO2æɱ»ŹÆ»ŅĖ®ĪüŹÕ£¬Éś³É·ĻŌüĪŖCaSO3£¬ĘųĢåaŹĒ²»Äܱ»¹żĮæŹÆ»ŅĖ®ĪüŹÕµÄN2”¢NO”¢CO£¬ĘųĢåaĶØČėæÕĘų£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗ“¦Ąķŗóµ½µÄNaNO2£¬ĶØČėæÕĘųµ«²»ÄܹżĮ棬·ńŌņµĆµ½ĻõĖįÄĘ£¬NaNO2Óėŗ¬ÓŠNH4+µÄČÜŅŗ·“Ӧɜ³ÉĪŽĪŪČ¾ĘųĢå£¬Ó¦Éś³ÉµŖĘų£¬ŌņĘųĢåbŗ¬ÓŠCO”¢N2£¬²¶»ń¼ĮĖł²¶»ńµÄĘųĢåÖ÷ŅŖŹĒCO£¬
¢Ł¹¤Ņµ·ĻĘųÖŠSO2æɱ»ŹÆ»ŅĖ®ĪüŹÕ£¬Éś³ÉCaSO3£¬ŅņĒāŃõ»ÆøĘ¹żĮ棬Ōņ·ĻŌüµÄ³É·ÖĪŖÖ÷ŅŖŗ¬ÓŠCa£ØOH£©2”¢CaSO3£¬¹Ź“š°øĪŖ£ŗCa£ØOH£©2”¢CaSO3£»
¢ŚÓÉ·ÖĪöæÉÖŖ£¬ĘųĢå1ŹĒ²»Äܱ»¹żĮæŹÆ»ŅĖ®ĪüŹÕµÄN2”¢NO”¢CO£¬ĘųĢåaĶØČėæÕĘų£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗ“¦Ąķŗóµ½µÄNaNO2£¬ĶØČėµÄæÕĘų²»ÄܹżĮ棬¹żĮæµÄæÕĘųÖŠµÄŃõĘų¾ßÓŠŃõ»ÆŠŌ£¬µĆµ½ĻõĖįÄĘ£¬
¹Ź“š°øĪŖ£ŗæÕĘų¹żĮ棬²æ·ÖµŖŌŖĖŲ×Ŗ»ÆĪŖNaNO3£¬NaNO2²śĮæ½µµĶ£»
¢ŪĘųĢåbŗ¬ÓŠCO”¢N2£¬¾²¶»ń¼ĮµĆµ½µŖĘųŗĶCO£¬Ėł²¶»ńµÄĘųĢåÖ÷ŅŖŹĒCO£¬·ĄÖ¹ĪŪČ¾æÕĘų£¬¹Ź“š°øĪŖ£ŗCO£»
¢ÜNaNO2Óėŗ¬ÓŠNH4+µÄČÜŅŗ·“Ӧɜ³ÉĪŽĪŪČ¾ĘųĢå£¬Ó¦Éś³ÉµŖĘų£¬·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗNH4++NO2-=N2”ü+2H2O£¬
¹Ź“š°øĪŖ£ŗNH4++NO2-=N2”ü+2H2O£»
£Ø2£©a£®Č”10mLøĆČÜŅŗÓŚŹŌ¹ÜÖŠµĪ¼ÓBa£ØNO3£©2ČÜŅŗ£¬¼ÓĻ”ĻõĖįĖį»Æŗó¹żĀĖµĆµ½0.03mol°×É«³Įµķ£¬øł¾ŻSO42-+Ba2+=BaSO4”żÖŖČÜŅŗÖŠŗ¬ÓŠSO42-ĪŖ0.04mol£»ĻņĀĖŅŗÖŠ¼ÓČėAgNO3ČÜŅŗĪ“¼ū³Įµķ²śÉś£¬ŌņČÜŅŗ֊ƻӊCl-£»
b£®ĮķČ”10mLøĆČÜŅŗÓŚŹŌ¹ÜÖŠ£¬µĪ¼ÓNaOHČÜŅŗ²śÉś°×É«³Įµķ£¬ŌņĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠAl3+£¬Al3++3OH-=Al£ØOH£©3”ż£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµ£¬ÖŖČÜŅŗÖŠŗ¬ÓŠAl3+ĪŖ0.015molŠčOH-ĪŖ0.045mol£¬ĒāŃõ»ÆÄĘČÜŅŗĢå»ż=30ml£¬ÅضČc=$\frac{0.045mol}{0.030L}$=1.5mol/L£¬µ±³ĮµķŌö¼Óµ½Ņ»¶ØĮæŗóæŖŹ¼²śÉśĘųĢ壬øł¾ŻNH4++OH-=NH3”ü+H2O½įŗĻĶ¼Ļó£¬ÖŖČÜŅŗÖŠŗ¬ÓŠNH4+ĪŖ0.03molŠčOH-ĪŖ0.03mol£»×īŗó³ĮµķĶźČ«ČܽāŹĒÓÉÓŚĒāŃõ»ÆĀĮÓėNaOH¼ĢŠų·“Ó¦£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl£ØOH£©3+OH-=AlO2-+2H2O£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµ£¬ŠčOH-ĪŖ0.015mol£»ŹµŃéÖŠŹ¹ÓƵÄNaOHČÜŅŗµÄ×ÜĪļÖŹµÄĮæĪŖ£ŗ0.045mol+0.03mol+0.015mol=0.09mol£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµÖŖ“ĖŹ±ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»ż=$\frac{0.09mol}{1.5mol/L}$=0.06L=60mL£¬
¢ŁŅĄ¾ŻÉĻŹö·ÖĪöæÉÖŖ£¬øĆ·ĻĖ®ÖŠŅ»¶Øŗ¬ÓŠµÄĄė×ÓÓŠ£ŗNH4+”¢Al3+”¢SO42-£¬Ņ»¶Ø²»ŗ¬Ģ¼ĖįøłĄė×Ó£¬½įŗĻµēŗÉŹŲŗć£ŗŃōĄė×ÓĖł“ųµēŗÉ×ÜŹżĪŖ£ŗ3n£ØAl3+£©+n£ØNH4+£©=3”Į0.015mol+0.03mol=0.06mol£¬ŅõĄė×ÓĖł“ųµēŗÉ×ÜŹż=2n£ØSO42-£©=2”Į0.04mol=0.08mol£¬ŌņŅ»¶Øŗ¬ÓŠNa+ĪļÖŹµÄĮæĪŖ0.02mol£¬
¹Ź“š°øĪŖ£ŗNa+”¢NH4+”¢Al3+”¢SO42-£»
¹Ź“š°øĪŖ£ŗK+”¢NH4+”¢Al3+”¢SO42-£»
¢ŚÅäÖĘ²½ÖčÓŠ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬Ņ»°ćĢģĘ½³ĘČ”ĒāŃõ»ÆÄĘ£¬°ŃĒāŃõ»ÆÄʵ¹ČėÉÕ±½ųŠŠČܽā£¬ĄäČ“ŗó×ŖŅʵ½100mLČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬Åä³É100mLČÜŅŗ£¬ĖłŅŌŠčŅŖµÄŅĒĘ÷ÓŠ²£Į§°ō”¢ĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢½ŗĶ·µĪ¹Ü”¢100mLČŻĮæĘ棬ĖłŅŌ»¹ŠčŅŖ²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢100mLČŻĮæĘ棬
¹Ź“š°øĪŖ£ŗ100mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
¢ŪŹµŃéÖŠŹ¹ÓƵÄNaOHČÜŅŗµÄ×ÜĪļÖŹµÄĮæĪŖ£ŗ0.045mol+0.03mol+0.015mol=0.09mol£¬½įŗĻĶ¼ĻóÖŠ³ĮµķµÄ±ä»Æ¹ŲĻµÖŖ“ĖŹ±ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»ża=$\frac{0.09mol}{1.5mol/L}$=0.06L=60mL£¬
¹Ź“š°øĪŖ£ŗ60£®
µćĘĄ ±¾Ģāæ¼²éĄė×ÓµÄĶʶĻ”¢Ąė×ÓŠŌÖŹ”¢Ąė×Ó·“Ó¦ĻÖĻó”¢Ąė×ÓµēŗÉŹŲŗćµÄ¼ĘĖćµČ£¬øł¾ŻĢāøÉĢį¹©µÄŹµŃéĻÖĻó½įŗĻĄė×ӵĊŌÖŹ½ųŠŠĶʶĻ£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ŅųŃĪ ŠŌÖŹ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ŃÕÉ« | °× | Ē³»Ę | °× | שŗģ | °× |
| Čܽā¶Č£Ømol•L-1£© | 1.34”Į10-6 | 7.1”Į10-7 | 1.1”Į10-8 | 6.5”Į10-5 | 1.0”Į10-6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĄÆÖņČ¼ÉÕ | B£® | »īŠŌĢæĪüø½±łĻäÖŠµÄŅģĪ¶ | ||
| C£® | ·ÖĄėŅŗĢ¬æÕĘųÖĘŃõĘų | D£® | ŗ£Ė®É¹ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 5 | B£® | 8 | C£® | 10 | D£® | 12 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | °¢Ė¾Ę„ĮÖ | B£® | ĒąĆ¹ĖŲ | C£® | Āé»ĘĖŲ | D£® | ĪøŹęĘ½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1HŗĶ2H | B£® | 14CŗĶ14N | C£® | 37ClŗĶ37Cl - | D£® | 56Fe2+ŗĶ56Fe3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČĘų£ŗK+”¢Na+”¢SiO32-”¢NO3- | B£® | ¶žŃõ»ÆĮņ£ŗNa+”¢NH4+”¢SO32-”¢C1- | ||
| C£® | Įņ»ÆĒā£ŗH+”¢K+”¢MnO4-”¢SO42- | D£® | °±Ęų£ŗK+”¢Na+”¢AlO2-”¢CO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1molH2OĖłŗ¬ÓŠµÄŌ×ÓŹżĪŖNA | |
| B£® | 32gO2ŗ¬ÓŠµÄŃõŌ×ÓŹżĪŖ2NA | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬11.2 LCl2 Ėłŗ¬µÄ·Ö×ÓŹżĪŖ0.5NA | |
| D£® | 1L0.1mol/LNaClČÜŅŗÖŠĖłŗ¬µÄNa+ŹżĪŖNA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com