
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
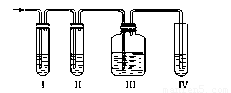
��1��д��ʵ��������ϩ�ķ�Ӧ����ʽ��
��2����װ�ÿ�ʢ�ŵ��Լ��ǣ���___________����___________����___________������Ũ��ˮ���������й��Լ����������ո��ڣ���
A��Ʒ����Һ B��NaOH��Һ C��ŨH2SO4 D������KMnO4��Һ
��3����˵����������������ڵ�������_______________________________��
��4��ȷ֤������ϩ��������_________________________________________��
��5��д�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
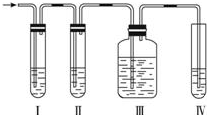 ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
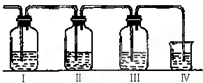 ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
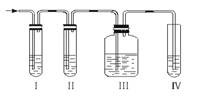
��1��д��ʵ��������ϩ�ķ�Ӧ����ʽ��
��2����װ�ÿ�ʢ�ŵ��Լ��ǣ���___________����___________����___________������Ũ��ˮ���������й��Լ����������ո��ڣ���
A��Ʒ����Һ B��NaOH��Һ C��ŨH2SO4 D������KMnO4��Һ
��3����˵����������������ڵ�������_______________________________��
��4��ȷ֤������ϩ��������_________________________________________��
��5��д�����з�����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13��)ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
(1)ʵ������ȡ��ϩ�Ļ�ѧ����ʽ_____________________����Ӧ����Ϊ_______��
(2)��װ�ÿ�ʢ�ŵ��Լ��Ǣ�______;��_____;��; _____��_______��(�������й��Լ����������ո���)
A.����KMnO4 B. Ʒ����Һ C. NaOH��Һ D. ŨH2SO4
(3)��˵����������������ڵ�������__________________________________��
(4)װ�â��п�ʼ��Ӧʱ�����ӷ���ʽ��______________________________��
(5)ʹ��װ�â��Ŀ����______________________________________________��
(6)ȷ֤������ϩ������_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ����������SO2��ijͬѧ�������ʵ����ȷ��������������к�����ϩ��SO2��
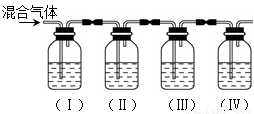
��1��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ��ǣ�I�� II�� III�� IV�� ���뽫�����й��Լ����������ո��ڣ���
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D����ˮ
��2����˵��SO2������ڵ������� ��
ʹ��װ��II��Ŀ���� ��
ʹ��װ��III��Ŀ���� ��
ȷ��������ϩ�������� ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ ������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com