�������ʽṹ�����ʡ���
��1������þ��������Ҫ����;��������ѧ��ά���?���������������л��ϳɷ�����;�㷺�ĸ������Լ����ٻ�ŵ������ѧ�����������Լ��Ľṹ��ʽ�ɱ�ʾΪRMgX�����ǽ���þ��±������Ӧ�IJ����Ƹ����Լ��������ѵ�ϡ��Һ���Ե�����ʽ���ڣ��������������ϣ���Ũ��Һ���Զ�������ڣ��ṹ��ͼ��
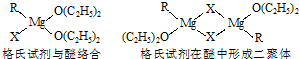
������2�ֽṹ�о�������λ����������Ϊ����λ�����á��������
��
����ԭ�Ӽ�ijɼ��ص㣬����Ԥ������ԭ��Mg���ӻ����Ϳ���Ϊ
��Mgԭ�ӵĺ�������Ų�ʽ�ɱ�ʾΪ
��
�����бȽ�����ȷ����
A����������ǿ����Mg��Al B����̬ԭ�ӵ�һ�����ܣ�Mg��Al
C�������ԣ�Mg��Al D�������ܣ�NaCl��MgCl
2��2����TiCl
4�������������þ���ȵõ��ѣ�TiCl
4+2Mg
Ti+2MgCl
2��TiԪ����Ԫ�����ڱ��е�λ����
����ԭ�ӵ���Χ�����Ų�ʽΪ
��
��TiCl
4�ڳ���������ɫҺ�壬��ˮ��ʪ��������ˮ���ð���̣���TiCl
4����
���ԭ�ӡ��������ӡ������ӡ������壮
�۶���������������ܽ�������Ⱦ���ȩ�������к������ת��Ϊ������̼��ˮ���ﵽ�������йؼ�ȩ������������̼��ˮ˵����ȷ����
��
A������B
3N
3H
6��Ϊ�ȵ�����
B����ȩ����������̼ԭ�Ӿ�����sp
2�ӻ�
C������������̼�ǷǼ��Է��ӣ�ˮ�ͼ�ȩ�Ǽ��Է���
D��ˮ�ķе�ȼ�ȩ�ߵö࣬����Ϊˮ���Ӽ����γ����
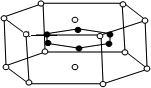
��3��2001�걨�������þ�γɵĻ�����ˢ���˽��������ﳬ���¶ȵ����¼����ͼ��ʾ��ĸû�����ľ���ṹ��Ԫ��þԭ�Ӽ��γ����������������������µ��滹����һ��þԭ�ӣ�������ԭ��λ�������ڣ���û�����Ļ�ѧʽ�ɱ�ʾΪ
A��MgB B��MgB
2 C��Mg
2B D��Mg
3B
2��

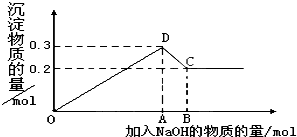 ��MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ�������NaOH�����ʵ�����mol�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����
��MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ�������NaOH�����ʵ�����mol�������ó��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�

