
��10�֣������õķ϶�ӰҺ�к���Na����[Ag(S2O3)2]3����Br�������ӡ�ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������塣����֪��4H��+2 [Ag(S2O3)2]3��= Ag2S��+3S��+3SO2��+SO42��+2H2O��
�Ų������������ �� ����������Ҫ����Ҫ���������� �� ��
�Ƽ���п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ ����
��Һ��B�г�����Br��������SO42����������Һ�д���SO42���IJ����� �� ��
�Ȳ����ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ����� �� ��
��ͨ�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ �� ��
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ̩����ѧ������ѧ���л�ѧ�Ծ� ���ͣ������
(12��) (12��) �����õķ϶�ӰҺ�к���Na����[Ag(S2O3)2]3����Br�������ӡ�ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������塣����֪�� 4H��+2 [Ag(S2O3)2]3�� = Ag2S��+3S��+3SO2��+SO42��+2H2O��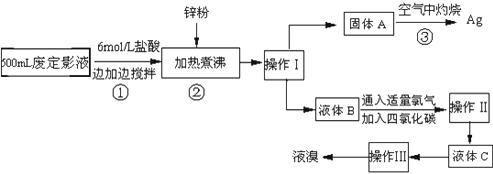
��1��������������� �� ����������Ҫ����Ҫ���������� �� ��
��2������п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ ����
��3��Һ��B�г�����Br��������SO42����������Һ�д���SO42���IJ����� �� ��
��4�������ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ����� �� ��
��5��ͨ�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡʦ���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣������õķ϶�ӰҺ�к���Na����[Ag(S2O3)2]3����Br��������.ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е�������.
����֪��4H��+2 [Ag(S2O3)2]3�� = Ag2S��+3S��+3SO2��+SO42��+2H2O��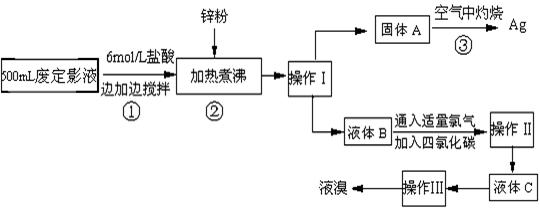
��1��������������� ����������Ҫ����Ҫ����������  .
.
��2������п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ .
��3��Һ��B�г�����Br��������SO42����������Һ�д���SO42���IJ�����
.
��4�������ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ����� .
��5�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ����� .
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com