
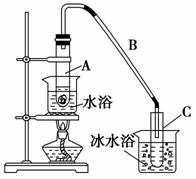
ij»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ(ČēĶ¼)£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©”£
ŅŃÖŖ£ŗ
| ĆܶČ/(g”¤cm£3) | ČŪµć/”ę | ·Šµć/”ę | ČܽāŠŌ | |
| »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® |
| »·¼ŗĻ© | 0.81 | ”Ŗ103 | 83 | ÄŃČÜÓŚĖ® |
(1)Öʱø“ÖĘ·
½«12.5 mL»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ¼ÓČė1 mLÅØĮņĖį£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ________£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠµÄ×÷ÓĆŹĒ________________________________________________________________”£
¢ŚŹŌ¹ÜCÖĆÓŚ±łĖ®Ō”ÖŠµÄÄæµÄŹĒ______________________________________
__________________________________ӣ
(2)Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£¼ÓČė±„ŗĶŹ³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ________²ć(Ģī”°ÉĻ”±»ņ”°ĻĀ”±)£¬·ÖŅŗŗóÓĆ________(ĢīČė±ąŗÅ)Ļ“µÓ”£
a£®KMnO4ČÜŅŗ””b£®Ļ”H2SO4””c£®Na2CO3ČÜŅŗ
¢ŚŌŁ½«»·¼ŗĻ©°“ČēĶ¼×°ÖĆÕōĮó£¬ĄäČ“Ė®“Ó________æŚ½ųČė”£ÕōĮóŹ±ŅŖ¼ÓČėÉśŹÆ»Ņ£¬ÄæµÄŹĒ______________________________________________________
________________________________________________________________ӣ
¢ŪŹÕ¼Æ²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚ________×óÓŅ£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棬æÉÄܵÄŌŅņŹĒ________”£
a£®ÕōĮ󏱓Ó70 ”ęæŖŹ¼ŹÕ¼Æ²śĘ·
b£®»·¼ŗ“¼Źµ¼ŹÓĆĮæ¶ąĮĖ
c£®Öʱø“ÖĘ·Ź±»·¼ŗ“¼Ėę²śĘ·Ņ»ĘšÕō³ö
(3)ŅŌĻĀĒų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬ŗĻĄķµÄŹĒ________”£
a£®ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ””b£®ÓĆ½šŹōÄĘ””c£®²ā¶Ø·Šµć
½āĪö””½ā“š±¾ĢāæÉĮŖĻµæĪ±¾ÖʱøŅŅĖįŅŅõ„µÄŹµŃé×°ÖĆ”£(1)“ÖĘ·µÄÖʱø¼ČŅŖ·ĄÖ¹·“Ó¦Īļ±©·Š£¬ÓÖŅŖ·ĄÖ¹Éś³ÉĪļ»Ó·¢”£
(2)¾«Ę·µÄÖʱø¹Ų¼üŌŚÓŚ³żŌÓ£¬“ĖĪŹÉę¼°µ½·ÖŅŗŗĶÕōĮ󔣻·¼ŗĻ©ĆܶȱČĖ®Š”£¬ŌŚÉĻ²ć£¬Ļ“µÓŹ±Ń”ÓĆKMnO4ČÜŅŗ»įŃõ»Æ»·¼ŗĻ©£¬ÓÖŅņ“ÖĘ·ÖŠ»ģÓŠÉŁĮæĖįŠŌĪļÖŹ£¬Ļ“µÓŹ±²»ÄÜŌŁÓĆĻ”ĮņĖį£¬ŠčÓĆNa2CO3ČÜŅŗ½«¶ąÓąµÄĖį³żµō”£ĘäÖŠµÄĖ®·ÖæÉÓĆÉśŹÆ»Ņ³żČ„”£ÓÉÓŚ»·¼ŗ“¼µÄ·Šµć½ĻµĶ£¬Öʱø“ÖĘ·Ź±Ėę²śĘ·Ņ»ĘšÕō³ö£¬µ¼ÖĀ²śĮæµĶÓŚĄķĀŪÖµ”£
(3)Ēų·Ö¾«Ę·Óė“ÖĘ·²»ÄÜŃ”ÓĆKMnO4£¬ŅņĪŖ¶žÕß½Ōæɱ»KMnO4Ńõ»Æ£»ÓÉÓŚ“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼µČ£¬æÉÓėÄĘ×÷ÓĆ²śÉśĘųĢ壬¹ŹæÉÓĆNa¼ÓŅŌĒų·Ö£»²ā¶Ø·ŠµćŌņÄÜŗÜŗƵÄĒų·Ö¶žÕß”£
“š°ø””(1)¢Ł·ĄÖ¹ŅŗĢ屩·Š””ĄäÄż””¢Ś·ĄÖ¹»·¼ŗĻ©»Ó·¢””(2)¢ŁÉĻ””c””¢Śg””ĪüŹÕĖ®·Ö£¬±ćÓŚÕōĮó³öøü“æ¾»µÄ²śĘ·””¢Ū83 ”ę””c””(3)b”¢c


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĪļÖŹA”¢B”¢C”¢D”¢E”¢FŌŚŅ»¶ØĢõ¼žĻĀµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£¬øĆĮłÖÖĪļÖŹµÄŃęÉ«·“Ó¦¾ł³Ź»ĘÉ«”£
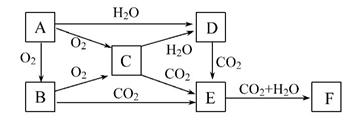
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗA”””””””””¢D”””””””””¢F”””””””””£
£Ø2£©A”¢B”¢C”¢DĖÄÖÖĪļÖŹ·Ö±š³¤ĘŚ±©Ā¶ŌŚæÕĘųÖŠ£¬Ęä×īÖÕ²śĪļĪŖ””””””””””””£¬ĘäÖŠ±äÖŹ¹ż³ĢÖŠÓŠŃõ»Æ»¹Ō·“Ó¦·¢ÉśµÄĮ½ÖÖĪļÖŹŹĒ(ĢīŠ“ĪļÖŹ¶ŌÓ¦µÄ»ÆѧŹ½)”” ”¢ ”£
£Ø3£©½«C¼ÓČėCuSO4ČÜŅŗÖŠ£¬·¢Éś·“Ó¦µÄ×Ü»Æѧ·½³ĢŹ½ĪŖ”” ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚT”ꏱ£¬Ä³NaOHĻ”ČÜŅŗÖŠc(H£«)£½10£a mol”¤L£1£¬c(OH£)£½10£b mol”¤L£1£¬ŅŃÖŖa£«b£½12”£ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČėpH£½cµÄŃĪĖį(T”ę)£¬²āµĆ»ģŗĻČÜŅŗµÄ²æ·ÖpHČēĻĀ±ķĖłŹ¾£ŗ
| ŠņŗÅ | NaOHČÜŅŗĢå»ż | ŃĪĖįĢå»ż | ČÜŅŗpH |
| ¢Ł | 20.00 | 0.00 | 8 |
| ¢Ś | 20.00 | 20.00 | 6 |
¼ŁÉčČÜŅŗ»ģŗĻĒ°ŗóµÄĢå»ż±ä»ÆŗöĀŌ²»¼Ę£¬ŌņcĪŖ(””””)
A.1 B£®4
C£®5 D£®6
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃé·½°ø²»ŗĻĄķµÄŹĒ
(”””” )
A£®ÓƱ„ŗĶNa2CO3ČÜŅŗ³żČ„ŅŅĖįŅŅõ„ÖŠ»ģÓŠµÄŅŅĖįµČ
B£®·ÖĄė±½ŗĶĻõ»ł±½µÄ»ģŗĻĪļ£¬æÉÓĆÕōĮó·Ø
C£®æÉÓƱ½½«äå“Óäå±½ÖŠŻĶČ”³öĄ“
D£®æÉÓĆĖ®Ą“¼ų±š±½”¢ŅŅ“¼”¢ĖÄĀČ»ÆĢ¼
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
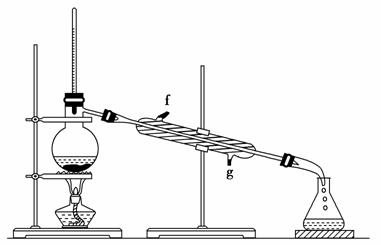
ĻÖÄā·ÖĄėŅŅĖįŅŅõ„”¢ŅŅĖįŗĶŅŅ“¼µÄ»ģŗĻĪļ£¬ĻĀĶ¼ŹĒ·ÖĄė²Ł×÷²½ÖčĮ÷³ĢĶ¼”£ĒėŠ“³öĶ¼ÖŠŌ²ĄØŗÅÄŚŹŹµ±µÄŹŌ¼Į£¬·½ĄØŗÅÄŚĖłÓƵķ֥ė·½·Ø£¬·½æņÄŚĖł·ÖĄėµÄÓŠ¹ŲĪļÖŹµÄ»ÆѧŹ½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹Ų½ŗĢåŗĶČÜŅŗµÄ±Č½ĻÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ČÜŅŗ³ŹµēÖŠŠŌ£¬½ŗĢå“ųÓŠµēŗÉ
B£®ČÜŅŗæÉĶø¹żĀĖÖ½£¬½ŗĢå²»æÉĶø¹żĀĖÖ½
C£®ČÜŅŗÖŠĶعż¹āŹųƻӊĢŲŹāĻÖĻ󣬽ŗĢåÖŠĶعż¹āŹųÓŠ¶”“ļ¶ūĻÖĻó
D£®½ŗĢåÓėČÜŅŗµÄ±¾ÖŹĒų±šŹĒ½ŗĢåÓŠ¶”“ļ¶ūĻÖĻó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲH2SO4µÄµēĄė·½³ĢŹ½£¬ÕżČ·µÄŹĒ(””””)
A£®H2SO4===H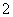 £«SO
£«SO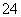 B£®H2SO4===2H£«£«S
B£®H2SO4===2H£«£«S 4
4
C£®H2SO4===2H£«£«S6£«£«4O2£ D£®H2SO4===2H£«£«SO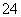
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻąĶ¬ĪĀ¶ČĻĀ£¬ŌŚĢå»żĻąµČµÄČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ·¢ÉśæÉÄę·“Ó¦£ŗ
N2(g)£«3H2(g) 2NH3(g) ”÷H£½£92.4 kJ/mol”£
2NH3(g) ”÷H£½£92.4 kJ/mol”£
ŹµŃé²āµĆĘšŹ¼”¢Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ±ķ£ŗ
| ČŻĘ÷±ąŗÅ | ĘšŹ¼Ź±ø÷ĪļÖŹĪļÖŹµÄĮæ/mol | Ę½ŗāŹ±·“Ó¦ÖŠµÄÄÜĮæ±ä»Æ | ||
| N2 | H2 | NH3 | ||
| ¢Ł | 1 | 3 | 0 | ·Å³öČČĮæa kJ |
| ¢Ś | 2 | 3 | 0 | ·Å³öČČĮæb kJ |
| ¢Ū | 2 | 6 | 0 | ·Å³öČČĮæc kJ |
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A£®·Å³öČČĮæ¹ŲĻµ£ŗa < b < 92.4 B£®ČżøöČŻĘ÷ÄŚ·“Ó¦µÄĘ½ŗā³£Źż£ŗ¢Ū>¢Ł>¢Ś
C£®“ļĘ½ŗāŹ±°±ĘųµÄĢå»ż·ÖŹż£ŗ¢Ł>¢Ū D£®N2µÄ×Ŗ»ÆĀŹ£ŗ¢Ś>¢Ł>¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éÖŠ£¬ĆæÖÖµē½āÖŹČÜŅŗµē½āŹ±Ö»Éś³ÉĒāĘųŗĶŃõĘųµÄŹĒ(””””)
A£®HCl”¢CuCl2”¢Ba(OH)2
B£®NaOH”¢CuSO4”¢H2SO4
C£®NaOH”¢H2SO4”¢Ba(OH)2
D£®NaBr”¢H2SO4”¢Ba(OH)2
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com