
����Ŀ��ij��ѧ��ȤС����ʵ���ҴӺ���������ȡ�Ⲣ�Ʊ�KI���塣��ش���������
(1)��ˮ��Һ����ȡ�����ѡ�õ��Լ���____________��(�����)
A���ƾ� B��CCl4 C����ϩ D��ֱ������
(2)KI������Ʊ���ʵ��װ����ͼ��

ʵ�鲽������
i������0.5mol��L1��KOH��Һ��
i��������ƿ�м���12.7g����I2��250mL 0.5mol��L1��KOH��Һ������������ȫ�ܽ⡣
����ͨ����Һ©����Ӧ�����Һ�еμ��������ᣬ��ַ�Ӧ��HCOOH������ΪCO2������KOH��Һ��pH��9~10����������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ������KI��Ʒ8.3g����ش��������⣺
������0.5mol��L1 KOH��Һʱ�����в���������õ���ҺŨ��ƫ�ߵ���_____(�����)��
A�������Ϸֱ����������ȵ�ֽƬ�����KOH����
B��KOH������Ʒ�л���K2O2
C�������õĹ�������ձ����ܽ�δ����ȴֱ��ת��������ƿ
D��δϴ���ձ���������ֱ��������ƿ�м�ˮ����
E������ʱ���ӿ̶���
�ڲ��袢��I2��KOH��Һ��Ӧ���ɵ���������ͻ�ԭ��������ʵ���֮��Ϊ1��5����д����������Ļ�ѧʽ��____________��
�۲��袣������Һ�еμ���������ʱ�������___________��(�a����b����a��b��)
��ʵ���У�����HCOOH����������ԭ��Ӧ�����ӷ���ʽΪ____________________��
��ʵ����KI�IJ���Ϊ________________%
���𰸡�BD BC KIO3 b 3HCOOH +IO3-===I-+3CO2��+ 3H2O 50
��������
��1����ˮ��Һ����ȡ�⣬��Ϊ��ȡ��������Ҫ���㣺��������ȡ�����ܽ�Ƚϴ���ȡ����ԭ�ܼ������ʾ�����Ӧ����ȡ����ԭ�ܼ������ܡ�
A.�ƾ����ˮ����Ӧ������ˮ���ܣ��ʲ�ѡA��
B.���Ȼ�̼���ˮ����Ӧ����ˮ�����ܣ��ҵ������Ȼ�̼�е��ܽ�ȸ���ѡB��
C.��ϩ���Ժ͵ⷴӦ����������ȡ�����ʲ�ѡC��
D.ֱ�����͵���Ҫ�ɷ��������뻷���������ˮ����Ӧ����ˮ�����ܣ��ҵ������е��ܽ�ȸ���ѡD����С���Ϊ��BD��
��2����A.KOH���������ˮ�ԣ�����������ж�����̼��Ӧ����ֽƬ�ϳ���KOH���壬��ʹ��������KOH����ƫС��������õ���ҺŨ��ƫ�ͣ��ʲ�ѡA��
B.1molK2O2��ˮ��Ӧ����2molKOH��1molK2O2������Ϊ110g����2molKOH������Ϊ112g����KOH������Ʒ�л���K2O2��ʹ������Һ������KOH�����ʵ�����������õ���ҺŨ��ƫ�ߣ���ѡB��
C.��Һδ����ȴ��ע������ƿ����ȴ����Һ�����С������Ũ��ƫ��ѡC��
D.δϴ���ձ���������ֱ��������ƿ�м�ˮ���ݣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ��ʲ�ѡD��
E.����ʱ���ӿ̶��ߣ�������Һ�����ƫ������������Һ��Ũ��ƫ�ͣ��ʲ�ѡE����С���Ϊ��BC��
��I2��KOH��Һ��Ӧ��I2�ȱ������ֱ���ԭ�������жϻ�ԭ����ΪKI�������ɵ���������ͻ�ԭ��������ʵ���֮��Ϊ1��5�����ݵ�ʧ�����غ㣬���жϳ����������е�Ԫ�ػ��ϼ�Ϊ+5�ۣ����Ƴ���������ΪKIO3����С���Ϊ��KIO3��
�۲��袣������Һ�еμ���������ʱ��ʹ�õ��Ǻ�ѹ��Һ©��������ƽ��ѹǿ���ã����ֻ�����b��������ʹ����˳�����¡���С���Ϊ��b��
������Ϣ��֪���������������ǻ�ԭ����أ�HCOOH������ΪCO2�����ݵ�ʧ�����غ�͵���غ�д��HCOOH����������ԭ��Ӧ�����ӷ���ʽΪ��3HCOOH +IO3-===I-+3CO2��+ 3H2O����С���Ϊ��3HCOOH +IO3-===I-+3CO2��+ 3H2O��
��12.7gI2�����ʵ���Ϊ0.05mol��KOH�����ʵ���Ϊ0.125mol����![]() ��֪��KOH������KIO3��HCOOH��ԭҲ����KI����������0.05molI2������0.1molKI������Ϊ16.6g��������ʵ����KI�IJ���Ϊ
��֪��KOH������KIO3��HCOOH��ԭҲ����KI����������0.05molI2������0.1molKI������Ϊ16.6g��������ʵ����KI�IJ���Ϊ![]() ����С���Ϊ��50%��
����С���Ϊ��50%��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�����ͼ���������б�Ҫ����������գ�

��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ��
���ӣ������Ӻ��װ�ý�____________���缫��Ӧʽ��
п�壺_____________________��ͭ�壺_______________________��
��2����Bͼ�У���a,bΪ���Ե缫��ʹa������ͭ����b������_________________��
���Ա�Ҫ�����Ӻ�װ�ý�________________���缫��Ӧʽ��a����______________________ b����______________________������һ��ʱ���ֹͣ��Ӧ��������Һ����Һ��pHֵ__________�����ߡ����͡����䣩������һ������_________����Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������HA��HB��H2C�����ᡣ��������0.1mol��L��1NaOH��Һ�ֱ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1mol��L��1��HA��HB���������Һ���ζ���������Һ��pH������NaOH ��Һ����ı仯��ͼ��ʾ��

��1��a��ʱ����Һ����ˮ�������c(H+)=________mol��L��1��Ka(HB)=________��
��2��������I �ϵ�c���Ӧ����Һ�и�����Ũ���ɴ�С��˳��Ϊ________��b���Ӧ����Һ��c(HB)____c(B��)(����>����<������=��)��
��3����֪��������0.1mol��L��1��NaHC ��Һ�е��뼸��ʯ����Һ����Һ��ɺ�ɫ��
������ô���Һ��pH=1����NaHC�ĵ��뷽��ʽΪ_______________��
�����ڴ���Һ���ܼ�H2C ���ӣ������Һ��c(C2��)________c(H2C)(����>����<������=��)��
����H2C��һ������ΪH2C=H++ HC����������0.1mol��L��1H2C��Һ�е�c(H+ )=0.11mol��L��1����0.1mol��L��1NaHC��Һ�е�c(H+)________0.01mol��L��1(����>����<������=��)��
��4����֪�¶�ʱ��0.1 mol��L��1��ijһԪ��HB��ˮ���� 0.1% �������룬�ش����и����⣺
�ٸ���Һ��pH��________��
��HB�ĵ���ƽ�ⳣ��K��________��
����HB�������c(H��)ԼΪˮ�������c(H��)��________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
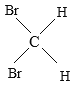
����Ŀ���������������ʣ�A�� O2��O3 B ��![]() Cl��
Cl��![]() Cl C ��CH4 ��C7H16 D��CH3CH2CH2CH3 ��
Cl C ��CH4 ��C7H16 D��CH3CH2CH2CH3 �� ��������E��
��������E��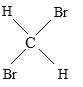 ��
��
��1��______���������ʻ�Ϊͬλ�أ�
��2��______���������ʻ�Ϊͬ�������壻
��3��______���������ʻ�Ϊͬϵ�
��4��______���������ʻ�Ϊͬ���칹�壻
��5��______����������ʵΪͬһ���ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⡣
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1��10��Ԫ���У���ѧ��������õ���__________��
��2���٢ڢ��У�����������ˮ���������ǿ����__________��
��3��10��Ԫ��������������ˮ���������ǿ����__________��
��4��Ԫ�آ���ɵĺ��Ǽ��Լ��ķ��ӵĵ���ʽ��__________��
��5�����֢١��ڵ�̼�����εļ�ʵ�鷽��__________��
��6���ٺ͢�����������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ__________��
��7���۵ĵ��������������ﷴӦ�ķ���ʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(�Ҷ���)������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ�����(��2���ᾧˮ)�Ĺ����������ң�

�ش��������⣺
��1��CO��NaOH��һ�������ºϳɼ����ơ������Ƽ�������Ļ�ѧ��Ӧ����ʽ�ֱ�Ϊ��__________________��_______________��
��2�����Ʊ������������ι��˲��������˲���������Һ��___________��������________�����˲���������Һ��____________��____________��������_______��
��3�����չ�������������Ŀ����______________��
��4�����˽�������������ֱ���������ữ�Ʊ����ᡣ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ��____________________��
��5���ᾧˮ�ϲ����Ʒ�Ĵ����ø�����ط��ⶨ��
���������Ʒ0.250 g����ˮ����0.0500 mol��L-1������KMnO4��Һ�ζ�����dz�ۺ�ɫ�����ʣ�����KMnO4��Һ15.00 mL����Ӧ�����ӷ���ʽΪ__________________��
��ʽ����ó�Ʒ�Ĵ���____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£����з�Ӧ��ˮ���������淴Ӧʱ��ı仯���Ʒ�����ͼ10 ����
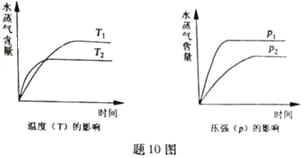
A. CO2(g)��2NH3(g)![]() CO(NH2)2(s)��H2O(g)����H��0
CO(NH2)2(s)��H2O(g)����H��0
B. CO2(g)��H2(g)![]() CO(g)��H2O(g)����H��0
CO(g)��H2O(g)����H��0
C. CH3CH2OH (g)![]() CH2=CH2(g)��H2O(g)����H��0
CH2=CH2(g)��H2O(g)����H��0
D. 2C6H5CH2CH3(g)��O2(g)![]() 2 C6H5CH=CH2(g)��2H2O(g)����H��0
2 C6H5CH=CH2(g)��2H2O(g)����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������dzµ�������������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
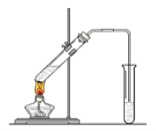
��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ��_____________
��2���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ��˳���ǣ�________________��
��3��Ũ����������ǣ���_________����________��
��4������̼������Һ����Ҫ������________��____ ��______��
��5��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ����___________��
��6����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������__________��
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ����_________��
��8���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ��������������Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)________��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ���������
�ݻ�����и����ʵ�Ũ�Ȳ��ٱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ӻ���CuCl2��FeCl2��FeCl3�Ĺ�ҵ��Һ�л���ͭ���Ʊ��Ȼ���������������£�
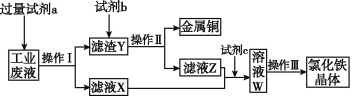
������˵����ȷ����
A. �Լ�a�������Լ�b��ϡ����
B. �����������������õ�������ȫ��ͬ
C. �Լ�c������,��Ӧ�����ӷ���ʽΪ2Fe2++Cl2![]() 2Fe3++2Cl-
2Fe3++2Cl-
D. ��KSCN��Һ�ɼ�����ҺW���Ƿ���Fe2+
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com