
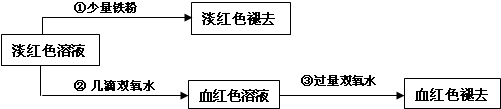
.ij�о���ѧϰС��Ϊ��̽��þ������ˮ��Ӧ�Ļ�����������������ʵ�飺�ٽ�þ��Ͷ�뵽��ˮ�У�δ�۲쵽�������ڽ�þ��Ͷ�뵽��ˮ�У��۲쵽ֻ�ǿ�ʼʱ���������������ݣ�����ˮ��������ɫ���۽�þ��Ͷ�뵽Һ���У�δ�۲쵽����������������þ�۵�Һ���еμӼ���ˮ���۲쵽��ĺ���ɫ�ܿ���ȥ�����й���þ������ˮ�ķ�Ӧ��������������ȷ����
A.þ��ֱֻ������ˮ�е��巴Ӧ B.þ��ֻ����ˮ�е��ᷴӦ
C.����������������������þ����ˮ��Ӧ�õ��� D.þ����ˮ�Ĵ������巢����Ӧ
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
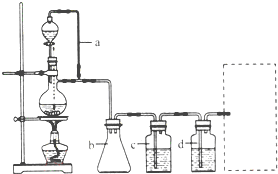 ��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
��2011?���ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�| һ������ |
| ||
| Ni���� |
| ||
| �� |
| ���� |
| ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
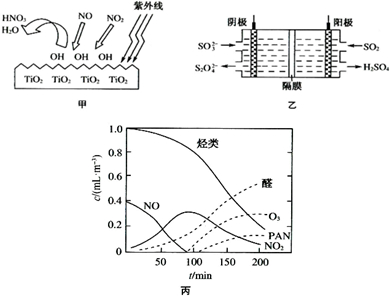
| O | 2- 4 |
| O | 2- 3 |
| O | 2- 4 |
| O | 2- 4 |
| O | 2- 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com