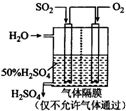
СђЫсБЛГЦЮЊЁАЙЄвЕжЎФИЁБЃЌзуМћЦфдкЙЄвЕЩњВњжаЕФживЊЕиЮЛЃЎРћгУДпЛЏбѕЛЏЗДгІНЋSO
2зЊЛЏЮЊSO
3ЪЧЙЄвЕЩњВњСђЫсЕФЙиМќВНжшЃЎвЛЖЈЮТЖШЯТЃЌЯђвЛИіДјЛюШћЕФЬхЛ§ЮЊ2LЕФУмБеШнЦїжаГфШы2.0mol SO
2ЃЈgЃЉКЭ1.0mol O
2ЃЈgЃЉЃЌЗЂЩњЗДгІЃКSO
2ЃЈgЃЉ+
O
2ЃЈgЃЉ?SO
3ЃЈgЃЉЁїH=-98kJ?mol
-1ЃЌЗДгІДяЕНЦНКтЪБЬхЛ§ЮЊ1.6LЃЎ
ЃЈ1ЃЉЗДгІДяЕНЦНКтЪБSO
2ЃЈgЃЉЕФЦНКтзЊЛЏТЪЮЊ
ЃЌИУЗДгІЕФЦНКтГЃЪ§K=
ЃЎ
ЃЈ2ЃЉвбжЊMn
2+ШмвКЯдЮоЩЋЃЎШєНЋзуСПSO
2ЭЈШыЫсЛЏЕФKMnO
4ШмвКжаЃЌГфЗжеёЕДКѓШмвКбеЩЋЭЪШЅЃЌгЩДЫЫЕУїSO
2Опга
ЃЈбЁЬюЁАЦЏАзадЁБЁЂЁАЛЙдадЁБЛђЁАбѕЛЏадЁБЃЉЃЌЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ
ЃЎ
ЃЈ3ЃЉЬьШЛКЃЫЎжажївЊКЌгаNa
+ЁЂK
+ЁЂCa
2+ЁЂCl
-ЁЂHCO
3-ЕШРызгЃЌЦфpHдМЮЊ8ЃЌдвђЪЧ
ЃЈгУРызгЗНГЬЪНБэЪОЃЉЃЎ
ЃЈ4ЃЉШєвдШчЭМЫљЪОзАжУЃЌгУЕчЛЏбЇдРэЩњВњСђЫсЃЌИУзАжУжаЭЈШыSO
2ЕФвЛМЋЕФЕчМЋЗДгІЪНЮЊ
ЃЎЗДгІЙ§ГЬжаШмвКРяЭЈЙ§ИєФЄЕФSO
42-ЕФвЦЖЏЗНЯђЪЧ
ЃЈЬюЁАгЩзѓЕНгвЁБЛђЁАгЩгвЕНзѓЁБЃЉЃЎ
Шєга96g SO
2ЃЈgЃЉБЛЭъШЋбѕЛЏЃЌРћгУЫљВњЩњЕФЕчФмЕчНтзуСПЕФCuSO
4ШмвКЃЈМйЩшФмСПзмРћгУТЪЮЊ80%ЃЉЃЌдђРэТлЩЯНЋВњЩњБъзМзДПіЯТO
2ЕФЬхЛ§ЪЧ
LЃЎ

 СђЫсБЛГЦЮЊЁАЙЄвЕжЎФИЁБЃЌзуМћЦфдкЙЄвЕЩњВњжаЕФживЊЕиЮЛЃЎРћгУДпЛЏбѕЛЏЗДгІНЋSO2зЊЛЏЮЊSO3ЪЧЙЄвЕЩњВњСђЫсЕФЙиМќВНжшЃЎвЛЖЈЮТЖШЯТЃЌЯђвЛИіДјЛюШћЕФЬхЛ§ЮЊ2LЕФУмБеШнЦїжаГфШы2.0mol SO2ЃЈgЃЉКЭ1.0mol O2ЃЈgЃЉЃЌЗЂЩњЗДгІЃКSO2ЃЈgЃЉ+
СђЫсБЛГЦЮЊЁАЙЄвЕжЎФИЁБЃЌзуМћЦфдкЙЄвЕЩњВњжаЕФживЊЕиЮЛЃЎРћгУДпЛЏбѕЛЏЗДгІНЋSO2зЊЛЏЮЊSO3ЪЧЙЄвЕЩњВњСђЫсЕФЙиМќВНжшЃЎвЛЖЈЮТЖШЯТЃЌЯђвЛИіДјЛюШћЕФЬхЛ§ЮЊ2LЕФУмБеШнЦїжаГфШы2.0mol SO2ЃЈgЃЉКЭ1.0mol O2ЃЈgЃЉЃЌЗЂЩњЗДгІЃКSO2ЃЈgЃЉ+

