
ŃĒĻõõ£ĀČ£ØNOCl£©ŹĒÓŠ»śĪļŗĻ³ÉÖŠµÄÖŲŅŖŹŌ¼Į£¬æÉÓÉNOÓėCl2ŌŚĶس£Ģõ¼žĻĀ·“Ó¦µĆµ½”£Ä³ŃŠ¾æŠŌѧĻ°Š”×éÄāŌŚŹµŃéŹŅĶØ·ē³÷ÖŠÖĘČ”ŃĒĻõõ£ĀČ£¬ĻČ²éµĆČēĻĀ׏ĮĻ£ŗ
¢Ł
| ·Ö×ÓŹ½ | ±šĆū | ČŪµć | ·Šµć | ČܽāŠŌ | ŠŌד |
| NOCl | Ńõ»ÆŃĒĻõõ£ | ØD64.5”ę | ØD5.5”ę | ČÜÓŚÅØĮņĖį | ŗģŗÖÉ«ŅŗĢå»ņ»ĘÉ«ÓŠ¶¾ĘųĢ壬¾ßÓŠ“Ģ¼¤¶ń³ō”£ÓöĖ®Éś³ÉµŖµÄŃõ»ÆĪļÓėĀČ»ÆĒā |
¢Ś2NO2+2NaOH=NaNO3+NaNO2+H2O
ĖęŗóĖūĆĒÄā¶ØĮĖČēĻĀŗĻ³É×°ÖĆŹ¾ŅāĶ¼
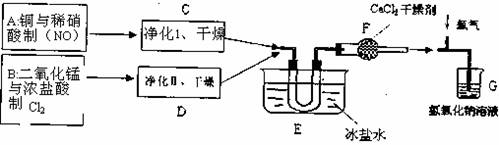
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öÖĘNOµÄĄė×Ó·½³ĢŹ½ £¬×°ÖĆӦє £Ø“ÓĻĀĶ¼ŅŅ”¢±ūÖŠŃ”£©£¬øĆ×°ÖĆŌŚŹ¹ÓĆĒ°£¬ŌõŃł¼ģŃéĘäĘųĆÜŠŌ£ØÖ»ÓŠÕōĮóĖ®£¬²»ÄÜŌŁĢķ¼ÓĘäĖūŅĒĘ÷»ņÓĆĘ·£© ”£
£Ø2£©»³ö¾»»Æ×°ÖĆIµÄ×°ÖĆĶ¼²¢±źĆ÷ĖłÓĆŹŌ¼Į ”£
£Ø3£©ĪŽĖ®ĀČ»ÆøʵÄ×÷ÓĆŹĒ £»Š“³öĀČ»ÆŃĒĻõõ£ÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø4£©ŌŚE×°ÖĆÖŠÄÜ擵½µÄĻÖĻó ”£
£Ø5£©Öøµ¼ĄĻŹ¦ČĻĪŖ×°ÖĆG²»ÄÜÓŠŠ§³żČ„ÓŠ¶¾ĘųĢ壬Š“³öÄćµÄ¼ū½āŗĶøĽų“ėŹ©
£Ø1£©3Cu+8H++2NOØD3=3Cu2++2NO”ü+4H2O£»±ū£»¹Ų±Õ»īČūaŗĶb£¬Ļņ·ÖŅŗĀ©¶·ÖŠ¼ÓŅ»¶ØĮæÕōĮóĖ®£¬“ņæŖa£¬ÖĮ·ÖŅŗĀ©¶·ÖŠÕōĮóĖ®ŅŗĆę²»ŌŁĻĀ½µŹ±¼ĒĻĀ“ĖĪ»ÖĆ£¬Ņ»¶ĪŹ±¼äŗó£¬ČēŅŗĆęĪ»ÖĆ²»ŌŁ±ä»Æ£¬ŌņĖµĆ÷ĘųĆÜŠŌŗĆ£¬·“Ö®Ōņ²»ŗĆ”£
£Ø2£© 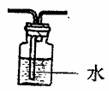
£Ø3£©·ĄÖ¹Ė®ÕōĘų½ųČėEµÄUŠĶ¹ÜÖŠÓėĀČ»ÆŃĒĻõõ£·“Ó¦”£
2NOCl+H2O=2HCl+N2O3£Ø»ņ2NOCl+H2O=2HCl+NO”ü+NO2”ü£©
£Ø4£©ŗģŗÖÉ«ŅŗĢåŗĶ»ĘÉ«ĘųĢå
£Ø5£©G×°ÖĆ²»ÄÜĪüŹÕUŠĶ¹ÜÖŠ»Ó·¢³öµÄĀČ»ÆŃĒĻõõ£”£½«F×°ÖĆ»»³ÉŅ»øöŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘæ£Ø»ņŌŚF”¢GÖ®¼ä¼ÓŅ»øöŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ棩



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com