
| A����֪a g��ϩ������ȼ��ʱ����1 mol CO2��Һ̬ˮ���ų�b kJ�����������ʾ��ϩȼ���ȵ��Ȼ�ѧ����ʽΪ2C2H4(g)��6O2(g)=4CO2(g)��4H2O(l) ��H����4b kJ·mol��1 |
B�����״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH(g)��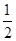 O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1 O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1 |
| C��H2(g)��ȼ������285.8 kJ·mol��1����2H2O(g)=2H2(g)��O2(g) ��H����571.6 kJ·mol��1 |
D�������ǵ�ȼ������2800kJ·mol��1����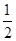 C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l) ��H����1400kJ·mol��1 C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l) ��H����1400kJ·mol��1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 (NH4)2 SO4��Һ����μ���0.2000 mol
(NH4)2 SO4��Һ����μ���0.2000 mol NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ����ͼ��ʾ�������ǻӷ�����
NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ����ͼ��ʾ�������ǻӷ�����
| A����a��ʾ��Һ�У�c(NH4+)��c(SO42-)��c(H+)��c(OH��) |
| B����b��ʾ��Һ�У�c(NH4+)��c(Na��)��c(H��)��c(OH��) |
| C����c��ʾ��Һ�У�c(SO42)+ c(H+)�� c(NH3��H2O )+ c(OH��) |
| D����d��ʾ��Һ�У�c(SO42)��c(NH3��H2O )��c(OH��)��c(NH4+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڼ�������������Դʱ��,���ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ |
| B���Ӵ�ú̿�Ŀ����ٶ�,����ú̿ȼ�ϵĹ�Ӧ��,�Ի���ʯ��Σ�� |
| C����������Ϣ��ҵ���й㷺Ӧ��,������µ���Ҫ�����Ƕ������� |
| D�����½ṹ�մɵ����裨Si3N4�����нϸߵ�Ӳ�Ⱥ���ĥ��,�������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��[(b��c)��125a]��100�� | B��[(b��2c)��125a]��100�� |
| C��[(b��c)��250a]��100�� | D��[(8b��8c)��a]��100�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH4(g)+2O2(g)=CO2(g)+2H2O(g)��H=-890.31kJ/mol |
| B��2H2(g)+ O2(g)= 2H2O(l)��H=-285.8kJ/mol |
| C��CO (g)+ H2O(g)= CO2(g)+ H2 (g)��H=+175.3kJ/mol |
| D��2CO (g)+ O2(g) = 2CO2(g)��H="-221" kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��ͼ�����Kw��Ĺ�ϵ��B>C>A=D=E |
| B��C��һ���Ǵ�ˮ |
| C��D������Ǵ�����Һ��E������Ǵ�������Һ |
| D��100��ʱ����pH=2��������pH=10��KOH�������Ϻ���Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO32�� | B��Fe3+ | C��HSO4�� | D��Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-542.7" kJ��mol-1 |
| B��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-1059.3" kJ��mol-1 |
C��N2H4(g)+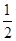 N2O4(g)= N2O4(g)=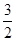 N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1 N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1 |
| D��2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ��H="-1076.7" kJ��mol-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com