




 ČżµćŅ»²āæģĄÖÖÜ¼Ę»®ĻµĮŠ“š°ø
ČżµćŅ»²āæģĄÖÖÜ¼Ę»®ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
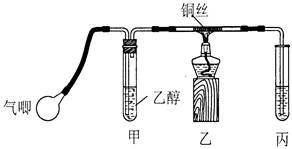
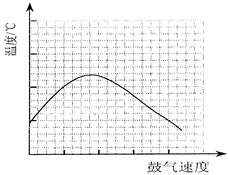
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
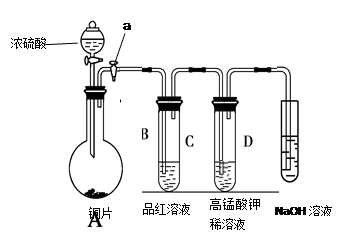
| ±ąŗÅ | ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč¢Ł | | ÓŠ°×É«³ĮµķÉś³É£¬Ö¤Ć÷“ż²āŅŗÖŠŗ¬SO42£”£ |
| ²½Öč¢Ś | | |
| ²½Öč¢Ū | | |
| ”” | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
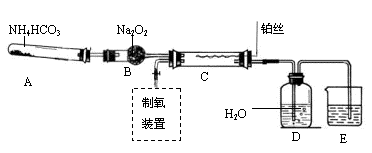
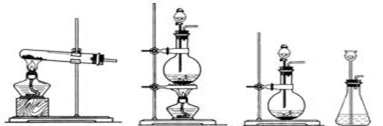
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
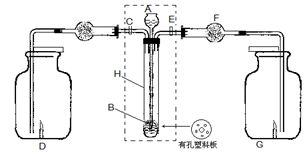
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
 l3ČÜŅŗČܽāAgµÄ·“Ó¦øüæģøüĶźČ«£¬Ēė½āŹĶĘäŌŅņ£ŗ””””””””””””””””””””””””””
l3ČÜŅŗČܽāAgµÄ·“Ó¦øüæģøüĶźČ«£¬Ēė½āŹĶĘäŌŅņ£ŗ””””””””””””””””””””””””””| ŹµŃé²½Öč£Ø²»ŅŖĒ󊓾ßĢå²Ł×÷¹ż³Ģ£© | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ¢Ł ¢Ś ”” | ČōŅų¾µĻūŹ§£¬¼ŁÉč2³ÉĮ¢”£ ČōŅų¾µ²»ĻūŹ§£¬¼ŁÉč2²»³ÉĮ¢”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
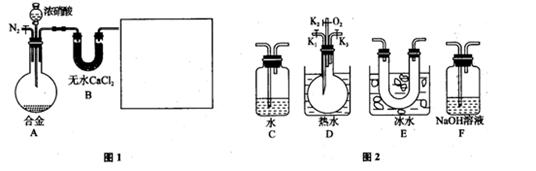
 _________________(2·Ö).
_________________(2·Ö).²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
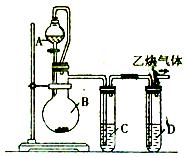
 »Æѧ·½³ĢŹ½£ŗ__________________________”£
»Æѧ·½³ĢŹ½£ŗ__________________________”£ ____________”£
____________”£ ĪŖĮĖ²āĮæ²śÉśµÄŅŅČ²ĘųĢåµÄĢå»ż£¬øĆŠ”×éĶ¬Ń§Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾µÄĮ½ÖÖ×°ÖĆ”£ŌņӦєŌń×°ÖĆ_______
ĪŖĮĖ²āĮæ²śÉśµÄŅŅČ²ĘųĢåµÄĢå»ż£¬øĆŠ”×éĶ¬Ń§Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾µÄĮ½ÖÖ×°ÖĆ”£ŌņӦєŌń×°ÖĆ_______ __£ØĢī¢ń»ņ¢ņ£©”£
__£ØĢī¢ń»ņ¢ņ£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com