
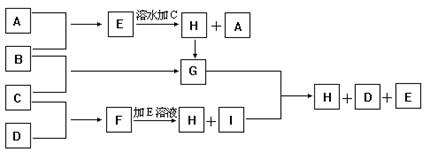

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®·Ē½šŹōŠŌ£ŗ |
B£®Ō×Ó°ė¾¶£ŗ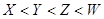 |
C£®×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ£ŗ |
| D£®µ„ÖŹY³£ĪĀĻĀÄÜČÜÓŚÅØĻõĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĪČ¶ØŠŌHCl>HBr>HI | B£®ĖįŠŌ HClO4>H2SO4 >H2CO3 |
| C£®Į£×Ó°ė¾¶ S2-<Cl-<K+<Ca2+ | D£®·Šµć I2>Br2>Cl2>F2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÉĻŹöĖÄÖÖŌŖĖŲµÄŌ×Ó°ė¾¶“óŠ”ĪŖW<X<Y<Z |
| B£®W”¢X”¢Y”¢ZŌ×ÓµÄŗĖĶā×īĶā²ćµē×ÓŹżµÄ×ÜŗĶĪŖ20 |
| C£®WÓėYæÉŠĪ³É¼Čŗ¬¼«ŠŌ¹²¼Ū¼üÓÖŗ¬·Ē¼«ŠŌ¹²¼Ū¼üµÄ»ÆŗĻĪļ |
| D£®ÓÉWÓėX×é³ÉµÄ»ÆŗĻĪļµÄ·Šµć×ܵĶÓŚÓÉWÓėY×é³ÉµÄ»ÆŗĻĪļµÄ·Šµć |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com