
Ϊ��Ԥ��������Ⱦ����β�������ۺ����ã�ij���᳧�ð�ˮ����β���е�SO2����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣
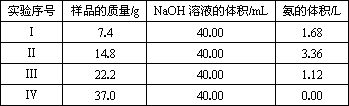
Ϊ�ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ4�ݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00 mL��������120 �����ң�ʹ����ȫ���ݳ�����NH4��2SO4��NH4HSO4����ֽ���¶Ⱦ�����200 �桳������й�ʵ���������£�����״���£�
| ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
| �� | 7.4 | 40.00 | 1.68 |
| �� | 14.8 | 40.00 | 3.36 |
| �� | 22.2 | 40.00 | 1.12 |
| �� | 37.0 | 40.00 | 0.00 |
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ_________________________________________��
��2���ɢ�������ֱ���Ʋ⣺��״����3.7 g ��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ__________L��
��3���Լ���û�����У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ__________________��
��4���������NaOH��Һ�����ʵ���Ũ��Ӧ��ѡ��__________�����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ____________________________________________________________��
��1��H++OH-====H2O��![]() +OH-
+OH-![]() NH3��+H2O ��2��0.84 (3)1��4 (4)�� 5 mol��L-1
NH3��+H2O ��2��0.84 (3)1��4 (4)�� 5 mol��L-1
��1����NH4��2SO4��NH4HSO4����Һ������ȫ����ģ����ԣ�NH4��2SO4��NH4HSO4���������м���NaOH��Һʱ�����������������ӷ�Ӧ����H++OH-====H2O��![]() +OH-
+OH-![]() NH3��+H2O��
NH3��+H2O��
��2���ɱ������ݿɿ���������Ʒ������7.4 g���ӵ�14.8 gʱ���ͷų������������1.68 L���ӵ�3.36 L���dzɱ������ӵģ�˵��NaOH��Һ��ʱ�������ġ����Կ��ƶϳ�����Ʒ������Ϊ3.7 gʱ�����ͷų����������ӦΪ1.68 L/2=0.84 L��
��3����I�������У�NH4��2SO4�����ʵ���Ϊx mol��NH4HSO4�����ʵ���Ϊy mol�����ݱ��е������У������������Ϊ14.8 gʱ����������NH3�����ʵ���Ϊ3.36 L/22.4 L��mol-1=0.15 mol��
��NH4��2SO4��2NH3 NH4HSO4��NH3
x mol 2x mol y mol y mol
![]() ��˷�����õ���x=0.025,y=0.1��
��˷�����õ���x=0.025,y=0.1��
���ԣ�������У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ0.025��0.1=1��4��
(4)�����������ݣ���������Ʒ����Ϊ22.2 gʱ���ų������������������Ʒ�����ɱ�����˵����ʱNaOH�������㣬�������жϳ�����ʱ��NaOH��������ȫ����H+���������˲��ֵ�![]() ����������ƷΪ37.0 gʱ���������ͷţ��������ʱNaOHȫ������Һ�е�H+���ġ������NaOH��Һ�����ʵ���Ũ��Ӧ��ѡ��������ݽ��м��㡣
����������ƷΪ37.0 gʱ���������ͷţ��������ʱNaOHȫ������Һ�е�H+���ġ������NaOH��Һ�����ʵ���Ũ��Ӧ��ѡ��������ݽ��м��㡣
��ϣ�3���ļ�������֪����22.2 g��Ʒ�к���NH4��2SO4ӦΪ0.025 mol��1.5=0.037 5 mol������NH4HSO4�����ʵ���Ϊ0.1 mol��1.5=0.15 mol��
����H+��NaOH������Һ�е�H+���ĵ�NaOHΪ0.15 mol��
����![]() ��NaOH��NH3,��Һ�е�
��NaOH��NH3,��Һ�е�![]() ���ĵ�NaOHΪ1.12 L/2.24 L��mol-1=0.05 mol��
���ĵ�NaOHΪ1.12 L/2.24 L��mol-1=0.05 mol��
���ԣ�NaOH�������ʵ���Ϊ0.15 mol+0.05 mol=0.2 mol��
NaOH��Һ�����ʵ���Ũ��Ϊ0.2 mol/0.04 L=5 mol��L-1��


 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�����ѵ��ֲᡡ���л�ѧ������1�����˽̰��¿α� �˽̰��¿α� ���ͣ�022
Ϊ��Ԥ��������Ⱦ����β�������ۺ����ã�ij���᳧�ð�ˮ����β���е�SO2����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2��(NH4)2SO4��NH4HSO4���壮
Ϊ�ⶨ����(NH4)2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ4�ݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00 mL��������120�����ң�ʹ��ȫ���ݳ�[(NH4)2SO4��NH4HSO4����ֽ���¶Ⱦ�����200��]������й�ʵ����������(��״����)��
(1)ʵ��������йط�Ӧ�����ӷ���ʽΪ________��
(2)�ɢ�������ֱ��Ԥ�⣺��״����3.7 g��Ʒ����ͬ��ʵ��ʱ�����ɰ������Ϊ________L��
(3)�û������(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ4�ݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00 mL��������120 �����ң�ʹ����ȫ���ݳ�����NH4��2SO4��NH4HSO4����ֽ���¶Ⱦ�����200 �桳������й�ʵ���������£�����״���£�
ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
�� | 7.4 | 40.00 | 1.68 |
�� | 14.8 | 40.00 | 3.36 |
�� | 22.2 | 40.00 | 1.12 |
�� | 37.0 | 40.00 | 0.00 |
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ_________________________________________��
��2���ɢ�������ֱ���Ʋ⣺��״����3.7 g ��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ__________L��
��3���Լ���û�����У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ__________________��
��4���������NaOH��Һ�����ʵ���Ũ��Ӧ��ѡ��__________�����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com