
 ��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������1��ʾ��
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������1��ʾ��| ������/kJ•mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ•mol-1 | 347 | 305 | 259 |
| ���Ӿ��� | NaCl | KCl | CaO |
| �����ܣ�kJ•mol-1�� | 786 | 715 | 3401 |
���� ��1��A��BΪ��������Ԫ�أ�AԪ�ص��ĵ����ܾ�����˵��Aԭ���������3�����ӣ���AΪAl��BԪ�ص��������ܾ�����˵��Bԭ���������2�����ӣ���BΪMg��
��2�������Ĺ��������е��������ڵ����ʷ�����һЩ��Ҫ��ѧ�����ܣ�ʹ��ѧ����ʽ���ѣ�
��ɵ����ʵ���İ�����ΪH2N-CH2-COOH��-CH2-�е�̼ԭ���ӻ������ĿΪ4��-COOH��̼ԭ���ӻ������ĿΪ3��
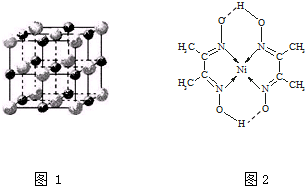
��3�����Ӿ���ľ�����Խ���۵�Խ�ߣ����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��ϱ��������жϣ�
MgO��NaCl�ṹ���ƣ�����ɫ����Mg2+�����ɫ��ΪO2-��������Mg2+Ϊ�о�������֮���ڽ��ҵȾ����Mg2+���ھ��������м䣻
��4�����������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�������ӵ������������ж�δ�ɶԵ��ӣ�
��5����ͬ�ǽ���ԭ��֮���γɼ��Լ���ͬ�ַǽ���Ԫ��ԭ��֮���γɷǼ��Լ���Oԭ����Hԭ��֮����������Nԭ����Ni֮���γ���λ����
��6��CO2���Ӻ���2���Ҽ���2���м���H2���Ӻ���1���Ҽ�����ϲμӷ�Ӧ��CO2��H2���ʵ������㣮
��� �⣺��1��A��BΪ��������Ԫ�أ�AԪ�ص��ĵ����ܾ�����˵��Aԭ���������3�����ӣ���AΪAl��BԪ�ص��������ܾ�����˵��Bԭ���������2�����ӣ���BΪMg��Al�������ϼ�Ϊ+3�ۣ�ͬ����������ҵ縺�����ʵ縺��Al��Mg��
�ʴ�Ϊ��+3������
��2���������е������ȵ����ʷ����еĻ�ѧ��C-C��C-N��C-S�ļ��ܴ���������������ʹ��Щ�����ѣ��Ӷ��ƻ������ʷ���
��ɵ����ʵ���İ�����ΪH2N-CH2-COOH��-COOH��̼ԭ���ӻ������ĿΪ3��-CH2-�е�̼ԭ���ӻ������ĿΪ4��������̼ԭ���ӻ�������sp2��sp3��
�ʴ�Ϊ���������е������ȵ����ʷ����еĻ�ѧ��C-C��C-N��C-S�ļ��ܴ���������������ʹ��Щ�����ѣ��Ӷ��ƻ������ʷ��ӣ�sp2��sp3��
��3�����Ӿ�������Ӱ뾶ԽС���������Խ�࣬������Խ��������۷е�Խ�ߣ�����TiN��MgO��MgO��CaO���ɱ������ݿ�֪CaO��KCl����TiN��MgO��CaO��KCl��
MgO��NaCl�ṹ���ƣ�����ɫ����Mg2+�����ɫ��ΪO2-��������Mg2+Ϊ�о�������֮���ڽ��ҵȾ����Mg2+���ھ��������м䣬����һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+����Ϊ12��
�ʴ�Ϊ��TiN��MgO��CaO��KCl��12��
��4��V2O5��V����������ȫ��ʧȥ��ɼ���CrO2��Crʧȥ4�����ӣ����ӵ���������Ϊ2��δ�ɶԣ����Ӻ�δ�ɶԵ���Խ�࣬�����Խ�����ʺ���¼�����ŷ�ԭ�ϵ���CrO2��
�ʴ�Ϊ��CrO2��
��5��Cԭ����Hԭ�ӡ�Nԭ��֮���γɼ��Լ���̼ԭ��֮���γɷǼ��Լ���Oԭ����Hԭ��֮����������Nԭ����Ni֮���γ���λ����û�����Ӽ�����������
��ѡ��AC��
��6������1mol CH4���ɣ�����1molCO2��4molH2�μӷ�Ӧ����CO2���Ӻ���2���Ҽ���2���м���H2���Ӻ���1���Ҽ�������ѦҼ�Ϊ2mol+4mol=6mol�����Ѧм�Ϊ2mol��
�ʴ�Ϊ��6��2��
���� �����Ƕ����ʽṹ�����ʵĿ��飬��Ŀ�����Ϊ�ۺϣ��漰��������֪ʶ��ע����վ����ܵıȽϣ�ע�������������������������ϵ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
 ��
�� ��
�� ���û���������ں��м��Լ�������ԡ��Ǽ��ԡ�����
���û���������ں��м��Լ�������ԡ��Ǽ��ԡ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S+O2�T2SO2����H=-269kJ/mol����Ӧ�ȣ� | |
| B�� | 2NO2��g���TO2��g��+2NO��g������H=+116.2kJ/mol����Ӧ�ȣ� | |
| C�� | C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g������H=-1367.0kJ/mol��ȼ���ȣ� | |
| D�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=+57.3kJ/mol���к��ȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 | 5 |
| V��NaOH��/mL���������� | 0.00 | 0.20 | 0.00 | 0.10 | 0.05 |
| V��NaOH��/mL���ն����� | 15.75 | 15.20 | 14.98 | 15.12 | 15.05 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com