

 NH4NO2��NaCl
NH4NO2��NaCl NH3����HNO2
NH3����HNO2 N2O3����H2O
N2O3����H2O 2N2��3H2O
2N2��3H2O| N2��H2������� | 5��1 | 3��1 | 1��1 | 1��3 | 1��5 |
| ��̪���ɫ����ʱ��/min | 8��9 | 7��8 | 6��7 | 3��4 | 9��10 |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
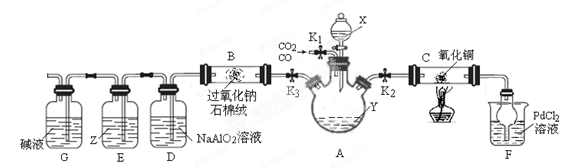
| ��� | ʵ����� | ʵ����������� | �� �� |
| �� | �� ��a g M�м���һ����ϡ���ᣬ��ֽ��裻 �� �����μ�ϡ����������, ��ַ�Ӧ. | �ٹ������Լ��٣� ����Ȼ��һ�������壬��Һ����ɫ | ��M��һ����Cu2O; ��M��һ����Cu. |
| �� | ����ʵ���������Һ���� ������ϴ�ӡ�������� | ��������Ϊ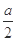 g g | MΪCu��Cu2O�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2CrO42������ɫ����2H+
2CrO42������ɫ����2H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
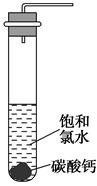
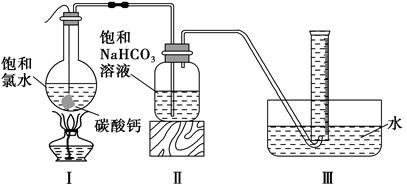
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
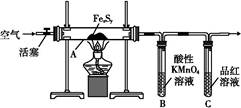
| �ζ����� | ������Һ ���/mL | ������Һ���/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.50 | 23.70 |
| 2 | 25.00 | 1.02 | 26.03 |
| 3 | 25.00 | 0.00 | 24.99 |
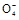 +2H2O+5SO2
+2H2O+5SO2 2Mn2++5S
2Mn2++5S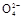 +4H+
+4H+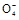 +6H++5H2C2O4
+6H++5H2C2O4 2Mn2++10CO2��+8H2O
2Mn2++10CO2��+8H2O| ��� | �¶�/�� | �ữ��H2C2O4 ��Һ/mL | KMnO4 ��Һ/mL | ��Һ�� ɫʱ��/s |
| 1 | 25 | 5.0 | 2.0 | 40 |
| 2 | 25 | 5.0(������������ ��ˮ��MnSO4��ĩ) | 2.0 | 4 |
| 3 | 60 | 5.0 | 2.0 | 25 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��װ�â�Ϊ�ų���ȡ��ˮ��ı��� |
| B��װ�â�Ϊ��Ȫʵ�� |
| C��װ�âۿ���������HCl���� |
| D����NH4ClΪԭ�ϣ�װ�âܿ������Ʊ�����NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ʵ�鲽�輰���� |
| A | �����������������Ƿ���� | ����  ��ɫ���� ��ɫ���� �������ܽ� �������ܽ� |
| B | ��ȡ������̽�������Ƿ����Ư���� | MnO2��ϡ���� ���� ���� ��ɫ ��ɫ |
| C | ֤����������H2O2�����Ա�I2ǿ | �⻯����Һ 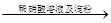 ��Һ����ɫ ��Һ����ɫ |
| D | ̽��Ũ�ȶ��ڻ�ѧƽ���Ӱ�� | FeCl3��KSCN�����Һ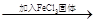 ��ɫ���� ��ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com