
(10��)��ʳ�γ���������Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ��������ᴿNaCl���������£�������Լ��Թ�����
 |
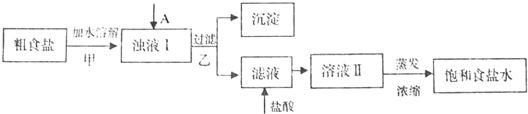
(1)������м�����Լ�A�� (�ѧʽ)��
(2)������У���ص����ӷ���ʽ
��
(3)����������벽��Ե������������pH�ٹ��ˣ�����ʵ����������Ӱ����
��
(4)���ᴿ��NaC1����100mL 1.0mol��L��NaCl��Һ�������������ձ�������������ͷ�ιܡ�ҩ���⣬����Ҫ (����������)��
(5)ʵ�����ᴿNaCl�Ĺ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ�������������������ʱ��ʹ�ò�������Ŀ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 4 |
2- 4 |
| ��ѧʽ | CaCO3 | CaSO4 | Ca��OH��2 | MgCO3 | Mg��OH��2 |
| Ksp | 2.8��10-9 | 9.1��10-6 | 1.0��10-4 | 6.8��10-6 | 1.8��10-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡտ�����и߶���һ���¿������ۺϻ�ѧ�Ծ����������� ���ͣ������
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
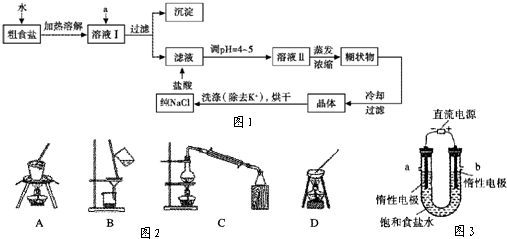
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ����ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ___��ֻ�ѧʽ����
��2�����ᴿ��NaCl����500mL 4.00 mol��L-1 NaCl��Һ������������ҩ�ס���������� �����������ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ����X�������۲쵽��ʵ�������� ��
��Y�缫�ϵĵ缫��ӦʽΪ ������õ缫��Ӧ����ķ����� ��
�����ռ���H2Ϊ2 L ����ͬ���������ռ���Cl2 ��������������� ���� 2 L��ԭ���� ��
2 L��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�߶���һ���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42-���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba(NO3)2��Һ����ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42-���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ___��ֻ�ѧʽ����
��2�����ᴿ��NaCl����500mL 4.00 mol��L-1 NaCl��Һ������������ҩ�ס���������� �����������ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����

�ٵ�����X���ϵĵ缫��ӦʽΪ ����X�������۲쵽��ʵ�������� ��
��Y�缫�ϵĵ缫��ӦʽΪ ������õ缫��Ӧ����ķ� ���� ��
�����ռ���H2Ϊ2 L ����ͬ���������ռ���Cl2 ��������������� ���� 2
L��ԭ����
��
2
L��ԭ����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com