
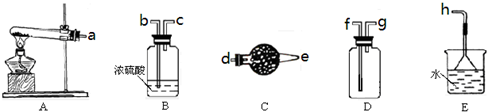
��12�֣���ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ�ã������豸���г̶ֹ�װ�þ���ȥ���������Ҫ��������и��⣨������װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ��a��bΪ������
��1��������ŨH2S04���ȣ���ˮ������CO��CO2������壬
![]()

ѡ���Ҫ����������һ��˳�������Ʊ�����֤�����е�CO��CO2��
����������˳���ǣ�����ţ���A��______��D��B_______��_________��
��ȷ����C02��װ���ǣ�____________�������ǣ�___________________��
��ȷ����CO��װ���ǣ�____________�������ǣ�___________________��
��2������Na2O2���Ƹ�����������������������ˮ�Ҵ�����֤���л����
��m�з�����Ӧ�Ļ�ѧ����ʽ��____________________________________��
��n���Լ��������ƣ�_____________���������ǣ�_______________������n���Լ��IJ����ǣ�___________________________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
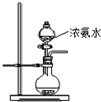 ��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ��
��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����8���¿������Ծ��������棩 ���ͣ�ʵ����
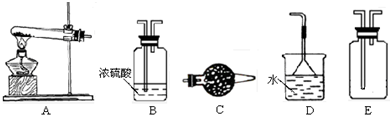
(14�֣���ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~100nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⡣

��1��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ ����ĸ����a�� �� �� �� ��h ����Aװ����ȡNH3�Ļ�ѧ��Ӧ����ʽΪ ��
��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ�� ��ѡ����ĸ��ţ���
A����ʯ�� B����ʯ�� C����ˮ�Ȼ��� D����ˮ����ͭ
E���ռ�
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��ƵĻ�ѧ����ʽ ��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������Ĵ��¿���ѧ�Ծ� ���ͣ�ʵ����
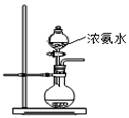
(11�֣���ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~100nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⡣

��1��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ ����ĸ����a�� �� �� �� ��h��
��Aװ����ȡNH3�Ļ�ѧ��Ӧ����ʽΪ
��2������ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ�� ��ѡ����ĸ��ţ���
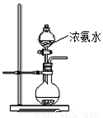
A����ʯ�� B����ʯ�� C����ˮ�Ȼ��� D����ˮ����ͭ E���ռ�
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼��� ʱ��Ӧ��ͨ��������� ����д��������̼��ƵĻ�ѧ����ʽ ��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com