
�������ƣ�NaN3����һ����ɫ���壬�����������Ʊ�����Ϊ2NaNH2��N2O��NaN3��NaOH��NH3��3NaNH2��NaNO3��NaN3��3NaOH��NH3����
�ش��������⣺
��1�������ڵ������У��縺������Ԫ����________����һ��������С��Ԫ����_______��
��2����̬��ԭ�ӵ�L������Ų�ͼΪ_______________��
��3����N3����Ϊ�ȵ�����ķ���Ϊ__________��д��һ�֣������ݼ۲���ӶԻ������ۣ�NO3���Ŀռ乹��Ϊ_____________��
��4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ_________________���������Ƶ�ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��_____________________________��
 ��5��N2O�е㣨��88��49������NH3�е㣨��33��34�����ͣ�����Ҫԭ����__________________��
��5��N2O�е㣨��88��49������NH3�е㣨��33��34�����ͣ�����Ҫԭ����__________________��
��6����ȫ���ҵ����ԭ��Ϊ6NaN3��Fe2O3?  3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2����
����������������������Ŀ֮��Ϊ________________________��
���������д��ڵĻ�ѧ������Ϊ__________________________��
��������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������
��1��������F����1�֣� ﮣ���Li����1�֣�
��2�� ��2�֣�
��2�֣�
��3��CO2��N2O��1�֣���ƽ�������Σ�1�֣�
��4�����Ӿ��壨1�֣���N3?+H2O HN3+OH?��2�֣�
HN3+OH?��2�֣�
��5��������֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ����2�֣�
��6��1��2��1�֣� ��������1�֣� ��112/NA��a3? g��cm-3��2�֣�����
��������
�����������1������ͬ����Ԫ�����ʵݱ�����жϣ������ڵ������У��縺������Ԫ���Ƿ�����һ��������С��Ԫ����ﮣ���2����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p3��L������Ų�ͼΪ ����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O
����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O HN3+OH����
HN3+OH���� ��5��N2O�е㣨��88��49������NH3�е㣨��33��34�����ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6�����������к��е�������������1�������ͺ�2����������Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����
��5��N2O�е㣨��88��49������NH3�е㣨��33��34�����ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6�����������к��е�������������1�������ͺ�2����������Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����
���㣺����Ԫ�������ɡ���������Ų�ʽ�����ӿռ乹�͡�����ṹ����ѧ�����жϼ��������㡣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
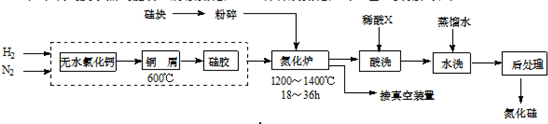
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧ�� | N-N | N=N | N��N | N-H | H-H |
| ����/kJ?mol-1 | 159 | 418 | 946 | 391 | 436 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������/g | 10.00 | 20.00 | 30.00 | 50.00 |
| Ũ�������ӵ�����/g | m | m | 1.29 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com