
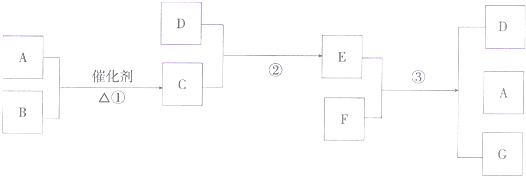
(8��)��Ӧ�٢�Ϊ��Ҫ�Ĺ�ҵ��Ӧ����Ϊ���ȼ��������Ҫ��Ӧ��EΪ����ɫ���壬PΪ����ɫ����I������Ϊ��ɫҺ�壬Ϊ��ʽ�Σ�J������Ԫ����ɣ���Է�������Ϊ100��O��Է�������Ϊ32�����ַ�Ӧˮ����ȥ��
w.w.w.k.s.5.u.c.o.
(1)д������O�Ľṹʽ ����C�ĵ���ʽ
(2)д����Ӧ�ٵ����ӷ���ʽ
(3)д����Ӧ�۵Ļ�ѧ����ʽ
(4)д����Ӧ�ܵĻ�ѧ����ʽ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ���� |
| �� |
| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)��ѧʽΪC2H6O�Ļ�����A�����������ʣ�
A+Na��Ӧ�����������ݣ�A+ CH3COOH��Ӧ�������й���ζ�IJ���
��1������������Ϣ���Ըû�������������ж���_____
A.һ�����С�OH B.һ�����С�COOH C.AΪ�Ҵ� D.AΪ��ȩ
��2����A���������Ϊ75����ˮ��Һ��������
��3��д��A�������Ļ�ѧ����ʽ
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ���Ľṹ��ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�����ʡ��ˮһ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��)��ѧʽΪC2H6O�Ļ�����A�����������ʣ�
A+Na��Ӧ�����������ݣ�A+ CH3COOH��Ӧ�������й���ζ�IJ���
��1������������Ϣ���Ըû�������������ж���_____
| A��һ�����С�OH | B��һ�����С�COOH | C��AΪ�Ҵ� | D��AΪ��ȩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��)��ѧʽΪC2H6O�Ļ�����A�����������ʣ�
A+Na��Ӧ�����������ݣ�A+ CH3COOH��Ӧ�������й���ζ�IJ���
��1������������Ϣ���Ըû�������������ж���_____
A.һ�����С�OH B.һ�����С�COOH C.AΪ�Ҵ� D.AΪ��ȩ
��2����A���������Ϊ75����ˮ��Һ��������
��3��д��A�������Ļ�ѧ����ʽ
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ���Ľṹ��ʽΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com