
̼���ơ���������Ӻ���(aNa2CO3��bH2O2)����Ư�ס�ɱ�����á�ʵ�����á����������Ʊ������ʵ�ʵ�鲽�����£�
��1����ȡ����̼�����ܽ���һ����ˮ�������ƿ�У��ټ��������ȶ���(MgCl2��Na2SiO3)��������ȡ�
��2����������30%��H2O2��Һ�ڽ���״̬�µ�����ƿ�У���15 �����ҷ�Ӧ1 h��
��3������Ӧ��Ϻ��ټ���������ˮ�Ҵ������á��ᾧ�����ˡ�����ò�Ʒ��
(1)��1���У��ȶ�����ˮ��Ӧ�������ֳ�����������仯ѧ����ʽΪ___________________________________________________________��
(2)��2���У���Ӧ����Ϊ15 �����ҿɲ�ȡ�Ĵ�ʩ��_____________________
___________________________________________________��
(3)��3���У���ˮ�Ҵ���������____________________________________��
(4)H2O2�ĺ����ɺ�����Ʒ�����ӡ��ֳ�ȡm g(Լ0.5 g)��Ʒ��������й�������ˮ���Ƴ�250 mL��Һ��ȡ25.0 mL����ƿ�У�����ϡ�����ữ������c mol��L��1 KMnO4��Һ�ζ����յ㡣
������250 mL��Һ����IJ����������ձ�������������Ͳ________��________��
�ڵζ��յ�۲쵽��������______________________________________��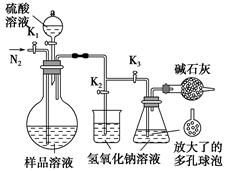
(5)��ģ�������ⶨ��Ʒ��̼���Ƶĺ�����װ������ͼ��ʾ(���Ⱥ̶�װ������ȥ)��ʵ�鲽�����£�
����1������ͼ��ʾ��װ���������װ�������ԡ�
����2��ȷ��ȡ(4)��������Һ50 mL����ƿ�С�
����3��ȷ��ȡ40.00 mLԼ0.2 mol��L��1 NaOH��Һ���ݣ��ֱ�ע���ձ�����ƿ�С�
����4������K1��K2���رջ���K3����ͨ�뵪��һ��ʱ��ر�K1��K2����K3������Һ©������ƿ�м���10 mL 3 mol��L��1������Һ��
����5����������ƿ�е�Һ����ڣ���������һ��ʱ�䡣
����6����K1�ٻ���ͨ�뵪��һ��ʱ�䡣
����7������ƿ�м������ָʾ������c1 mol��L��1 H2SO4����Һ�ζ����յ㣬����H2SO4����ҺV1 mL��
����8����ʵ�鲽��1��7�ظ����Ρ�
�ٲ���3�У�ȷ��ȡ40.00 mL NaOH��Һ����Ҫʹ�õ�������________��
�ڲ���1��7�У�ȷ�����ɵĶ�����̼������������Һ��ȫ���յ�ʵ�鲽����________(�����)��
��Ϊ�����Ʒ��̼���Ƶĺ��������貹���ʵ����______________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ijЩ������Ʊ������ʵ�ʵ��(ͼ�мг�װ����ʡ��)���밴Ҫ����գ�

��.̽�������백���ķ�Ӧ
(1)Ϊ��ȡ���ﰱ�����ɽ�װ��C��________(��װ�ñ��)���ӣ�װ��C�е���ƿ�ڹ�����ѡ��________��
a����ʯ�� b���Ȼ��� c������������ d����ʯ��
(2)װ��A��E��E���ӿ���ȡ�����������������������Eװ���ڵ�ҩƷ������________��
(3)װ��F������̽�������백��(��֪�����백���ɷ�����Ӧ��3Cl2��2NH3=N2��6HCl)�ķ�Ӧ��ʵ��ʱ����1��3���ر�2��������ƿ��ͨ��________��Ȼ��ر�1��3����2������ƿ�л���ͨ��һ��������һ�����塣ʵ��һ��ʱ�����ƿ�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����һ��ʵ�鷽�������ù����е�������________��
��.̽��ijЩ���ʵ�����
(1)����װ��A��E�������ʵ��Ƚ�Cl����Br���Ļ�ԭ��ǿ������֤�����۵�ʵ��������________��
(2)������װ��A��E������ϩ����ˮ��Ӧ��ʵ�飬�����װ��A���еĸĶ���________��
(3)��װ��B��C�ֱ���F��������H2S��SO2��Ӧ��ʵ�顣F����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ________________��F���ձ������������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
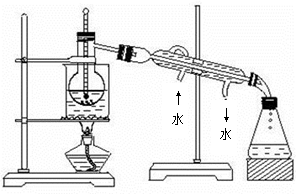
����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���________���֣�
��.��������ijɷֿ��ܺ���________���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1��������װ̽��ʵ���װ�ã����������ҵķ��������ӿڵ�����˳��Ϊ�١��������ޡ��ݡ�________��________��________��________���ڣ���ӿ���ţ���
��2����ʵ�����ʱB����Ͳû���ռ���ˮ����֤������________��ȷ��
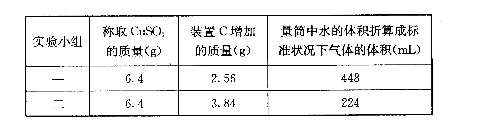
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
| ʵ�� С�� | ��ȡCuSO4 ������/g | װ��C���� ������/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
| һ | 6.4 | 2.56 | 448 |
| �� | 6.4 | 2.56 | 224 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϡ�����ʯ��Ҫ�ɷ�ΪCaCO3�������������ĺ����ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���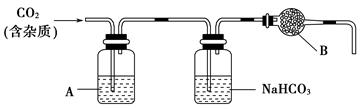
���������գ�
(1)��Ũ��������1��1(�����)��ϡ����(Լ6 mol��L��1)��Ӧѡ�õ�������________(����ĸ����ͬ)��
a���ձ������� b��������������c����Ͳ������ d������ƿ
(2)����װ���У�A��______��Һ��NaHCO3��Һ��������________________��
(3)����װ���У�B������________�������ʵ��õ�������ⶨCO2�ķ����������B����ʧЧ���ⶨ���________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(4)һ���Է�����ʯ��(������)��CaCO3��ʳ���е��ܳ��������۷���������ָ��֮һ���ⶨ�ܳ�������Ҫʵ�鲽��������£����顢���ء������ܽ�����ˡ�������ɡ���ȴ�����ء����ء�Ϊ�˽�ʯ����̼����ܳ���Ӧѡ�õ��Լ���________��
a���Ȼ�����Һ���� b��ϡ���ᡡ��c��ϡ���ᡡ�� d��������
(5)���ܳ����ⶨʵ���У�Ϊ�˻��ʯ����̼��Ƶ�����ܳ�����Ӧ���ܳ�________�����ܳ�________��
(6)�����ⶨʵ���У�����________��˵����Ʒ�Ѿ����ء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�ִ����к�����ɳ��Ca2����Mg2����Fe3����SO�����ʡ�ijͬѧ��ʵ����������������ִ����Ʊ����εķ�������(���ڳ������Լ��Թ���)��
��ش��������⣺
(1)Ϊ������ѡ����������(�ñ����ĸ��д)��________��
| A���ձ� | B���Թ� | C�������� | D����Һ©����E��©����F���ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�С���CuSO4�ֽ�����CuO��������3�ֿ����Խ��м���,��ͨ��ʵ��ȷ����������ʵ�����
(1)�����3�ֿ�����Ϊ����������;������������;��SO2��SO3��O2��
(2)Ϊȷ��SO2��SO3��O2�����ʵ���֮��,����ѡ����������������ʵ��,������������ҵķ������Ӹ�װ�á�˳��Ϊ:��������������B��(��֪SO3����Ũ����) 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����(H2C2O4)��һ�����ᣬ�ڹ�ҵ������Ҫ���á�ijͬѧ�����ϵ�֪�������и��������Բ����κ�̼���Ρ�������������ĥ��֭����ˮ���ݣ������˵õ���Һ������������CaCl2��Һ��������ɫ���������ˡ�ϴ�ӳ������ã�Ȼ������ɵij�������̽����
(1)��������Һ�������Ե�ԭ����_____________________________________��
(2)��ͬѧ�����ɵij������ж���̽����
������������衣
����1��ֻ����CaCO3��
����2���ȴ���CaCO3��Ҳ����CaC2O4��
����3��___________________________________________________________��
�ڻ��ڼ���2�����ʵ�鷽��������ʵ�顣�����±���д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ���ѡʵ���Լ���
1 mol��L��1 H2SO4��0.1 mol��L��1���ᡢ0.01 mol��L��1 KMnO4��Һ������ʯ��ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����������Թ��У�����_________________________________ ________________________________ | _______________________________ _______________________________ ˵����������CaCO3 |
| ����2��_________________________ ________________________________ | ________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ���ɵ�����ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO3��SO2��O2�е�һ�֡����ֻ����֡�ij��ѧ����С�����̽����ʵ�飬�ⶨ��Ӧ������SO3��SO2��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
��������롿
����I������ͭ���ȷֽ���������ijɷֿ���ֻ��SO3һ�֣�
���������ͭ���ȷֽ���������ijɷֿ���ֻ��_______���֡�
���������ͭ���ȷֽ���������ijɷֿ��ܺ���_______���֡�
��ʵ��̽����
��֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1����װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ�١��������ޡ��ݡ�____��_____��_____��______���ڡ�(��ӿ����)
��2����ʵ�����ʱװ��B����Ͳû���ռ���ˮ����֤������_______(�I������)��ȷ��
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ���������������Ҳ��
ͬ���������£�
��ͨ�����㣬�ƶϳ��ڵ�һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺_____________________________________________________________;
�ڶ�С�飺_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ԫ�ض��˵Ľ����dz���Ҫ�������ǴӺ������з�������ʵ⣨I2��������ͼ��
��1�����̢ٵ�ʵ�����������____________��____________��
��2�����������У�����������ԭ��Ӧ����_______�����ӷ���ʽΪ ��
��3�����̢۵�ʵ�����������___________��___________���ڴ˹����У��ɹ�ѡ�����
���ܼ���_______������ĸ���ţ���
| A���ƾ� | B��CCl4 | C���� | D������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com