
| A£® | ijĖįŠŌČÜŅŗÖŠÖ»ŗ¬ÓŠNa+£®CH3COO-£®H+£®OH-£¬ĻņøĆČÜŅŗÖŠ¼ÓČėŹŹĮæ°±Ė®£¬c£ØCH3COO-£©Ņ»¶Ø“óÓŚc£ØNa+£©Óė c£ØNH4+£©Ö®ŗĶ | |
| B£® | ³£ĪĀĻĀ½«0.01molCH3COONaŗĶ0.004molHClČÜÓŚĖ®£¬ÅäÖĘ³É0.5L»ģŗĻČÜŅŗ£®ČÜŅŗÖŠn£ØCH3COO-£©+n£ØOH-£©-n£ØH+£©=0.006mol | |
| C£® | ĻņĻõĖįÄĘČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖįµĆµ½µÄpH=5µÄ»ģŗĻČÜŅŗ£ŗc£ØNa+£©£¼c£ØNO3-£© | |
| D£® | ĪļÖŹµÄĮæÅضČĻąµČµÄNaFŗĶNaCNČÜŅŗÖŠŅõĄė×Ó×ÜŹżĻąµČ |
·ÖĪö A£®Čō¼ÓČėŹŹĮæ°±Ė®ÖĮ¼īŠŌ£¬ČÜŅŗÖŠ“ęŌŚc£ØCH3COO-£©+c£ØOH-£©=c£ØNa+£©+c£ØNH4+£©+c£ØH+£©£¬¾Ż“Ė·ÖĪö£»
B£®øł¾ŻµēŗÉŹŲŗć¼ĘĖćČÜŅŗÖŠn£ØCH3COO-£©+n£ØOH-£©-n£ØH+£©µÄÖµ£»
C£®pH=5µÄ»ģŗĻČÜŅŗ£¬ÄĘĄė×Ó”¢ĻõĖįøłĄė×Ó²»µēĄė”¢²»Ė®½ā£¬øł¾ŻĪļĮĻŹŲŗćÅŠ¶Ļ£»
D£®HFŗĶHCN¶¼ŹĒČõĖį£¬HFµÄĖįŠŌ±ČHCNµÄĖįŠŌĒ棬ĪļÖŹµÄĮæÅضČŗĶĢå»ż¾łĻąĶ¬µÄNaFŗĶNaCNĮ½ÖÖČÜŅŗÖŠŅõĄė×ÓĖ®½ā³Ģ¶ČF-£¼CN-£®
½ā“š ½ā£ŗA£®Čō¼ÓČėŹŹĮæ°±Ė®ÖĮ¼īŠŌ£¬ČÜŅŗÖŠ“ęŌŚc£ØCH3COO-£©+c£ØOH-£©=c£ØNa+£©+c£ØNH4+£©+c£ØH+£©£¬ŅņĪŖc£ØOH-£©£¾c£ØH+£©£¬Ōņc£ØCH3COO-£©£¼c£ØNa+£©+c£ØNH4+£©£¬¹ŹA“ķĪó£»
B£®øł¾ŻµēŗÉŹŲŗćæÉÖŖ£¬n£ØNa+£©+n£ØH+£©=n£ØCl-£©+n£ØOH-£©+n£ØCH3COO- £©£¬ĖłŅŌn£ØCH3COO-£©+n£ØOH-£©-n£ØH+£©=n£ØNa+£©-n£ØCl-£©=0.01mol-0.004mol=0.006mol£®¹ŹBÕżČ·£»
C£®ĻņĻõĖįÄĘČÜŅŗÖŠµĪ¼ÓĻ”ŃĪĖįµĆµ½µÄpH=5µÄ»ģŗĻČÜŅŗÖŠ£¬ŅĄ¾ŻĪļĮĻŹŲŗćµĆµ½c£ØNa+£©=c£ØNO3-£©£¬¹ŹC“ķĪó£»
D£®HFŗĶHCN¶¼ŹĒČõĖį£¬HFµÄĖįŠŌ±ČHCNµÄĖįŠŌĒ棬ČõĖįŌ½Čõ¶ŌÓ¦ĖįøłŅõĄė×ÓĖ®½ā³Ģ¶ČŌ½“ó£¬ĖłŅŌĪļÖŹµÄĮæÅضČŗĶĢå»ż¾łĻąĶ¬µÄNaFŗĶNaCNĮ½ÖÖČÜŅŗÖŠŅõĄė×ÓĖ®½ā³Ģ¶ČF-£¼CN-£¬Ė®½āČÜŅŗ³Ź¼īŠŌ£¬ŅõĄė×Ó×ÜŹż²»ĻąµČ£¬¹ŹD“ķĪó£»
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éĮĖµē½āÖŹČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”±Č½Ļ”¢µēŗÉŹŲŗć·ÖĪö”¢ĪļĮĻŹŲŗćÅŠ¶Ļ”¢ŃĪĄąĖ®½āŌĄķµČÖŖŹ¶µć£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®


 Ļ°Ģā¾«Ń”ĻµĮŠ“š°ø
Ļ°Ģā¾«Ń”ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1 molŅŗĢ¬ėĀŌŚ×ćĮæŃõĘųÖŠĶźČ«Č¼ÉÕÉś³ÉĖ®ÕōĘų£¬·Å³ö642kJµÄČČĮæ£ŗN2H4£Øl£©+O2£Øg£©ØTN2£Øg£©+2H2O£Øg£©”÷H=+642 kJ•mol-1 | |
| B£® | 12 gŹÆÄ«×Ŗ»ÆĪŖCOŹ±£¬·Å³ö110.5 kJµÄČČĮæ£ŗ2C£ØŹÆÄ«£¬s£©+O2£Øg£©ØT2CO£Øg£©”÷H=-110.5 kJ•mol-1 | |
| C£® | ŅŃÖŖ£ŗH2£Øg£©+$\frac{1}{2}$O2£Øg£©ØTH2O£Øl£©”÷H=-286 kJ•mol-1£¬Ōņ£ŗ2H2O£Øl£©ØT2H2£Øg£©+O2£Øg£©µÄ”÷H=+572 kJ•mol-1 | |
| D£® | ŅŃÖŖN2£Øg£©+3H2£Øg£©?2NH3£Øg£©”÷H=-92.4 kJ•mol-1£¬ŌņŌŚŅ»¶ØĢõ¼žĻĀĻņĆܱÕČŻĘ÷ÖŠ³äČė0.5 mol N2£Øg£©ŗĶ1.5 mol H2£Øg£©³ä·Ö·“Ó¦·Å³ö46.2 kJµÄČČĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±½·ÓŹĒŅ»ÖÖČõĖį£¬µĪ¼ÓÖøŹ¾¼Į»į±äÉ« | |
| B£® | ±½·ÓŌŚĖ®ČÜŅŗÖŠÄÜ°“ĻĀŹ½µēĄė£ŗ | |
| C£® | ±½·ÓÄĘĖ®ČÜŅŗĄļĶØČėCO2ĘųĢå×ÜŹĒÉś³ÉNaHCO3 | |
| D£® | ±½·ÓÓŠøÆŹ“ŠŌ£¬½¦ŌŚĘ¤·ōÉĻæÉÓĆ¾Ę¾«³åĻ“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cu£ØOH£©2 | B£® | Cu2£ØOH£©2SO4 | C£® | 3Cu£ØOH£©2•CuSO4 | D£® | Cu£ØOH£©2•3CuSO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±½ | B£® | CH3CHO | C£® | CH3COOH | D£® | CH2=CH-COOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗĖĖŲ | B£® | Ķ¬Ī»ĖŲ | C£® | ŌŖĖŲ | D£® | ĒāĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢĒĄą¶¼ÄÜ·¢ÉśĖ®½ā·“Ó¦ | |
| B£® | »ØÉśÓĶŌŚĖįŠŌ»ņ¼īŠŌĢõ¼žĻĀ·¢ÉśĖ®½āæÉÖʵƷŹŌķ | |
| C£® | µ°°×ÖŹČÜŅŗÖŠ£¬¼ÓČėÅصÄĮņĖįļ§ČÜŅŗÓŠ³ĮµķĪö³ö£¬¼ÓĖ®ŗó³ĮµķČܽā | |
| D£® | Č”ŹŹĮæµķ·ŪČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČėÉŁĮæĻ”ĮņĖį£¬¼ÓČČ3”«5min£¬ŌŁĄäČ“£¬µĪ¼Ó4”«6µĪĒāŃõ»ÆĶŠü×ĒŅŗ£¬¹Ū²ģÓŠĪŽ³öĻÖŗģÉ«³Įµķ£¬“Ó¶ųÅŠ¶Ļµķ·ŪŹĒ·ń·¢ÉśĮĖĖ®½ā·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 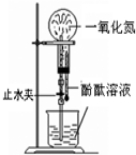 ÅēČŖŹµŃé | B£® | 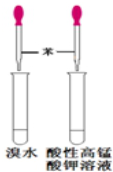 ŃéÖ¤±½ÖŠŹĒ·ńŗ¬ÓŠĢ¼Ģ¼Ė«¼ü | ||
| C£® | 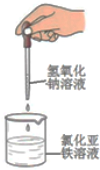 ÖʱøĒāŃõ»ÆŃĒĢś | D£® | 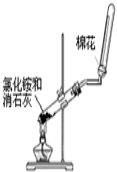 ŹµŃéŹŅÖĘČ”²¢ŹÕ¼Æ°±Ęų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
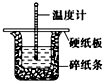 50mL 0.50mol•L-1ŃĪĖįÓė50mL 0.55mol•L-1NaOHČÜŅŗŌŚĶ¼Ź¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ
50mL 0.50mol•L-1ŃĪĖįÓė50mL 0.55mol•L-1NaOHČÜŅŗŌŚĶ¼Ź¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com