
���� I����1����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ����������������Һ���ѡ����ʹ������ƿ��
��2������m=CVM������Ҫ��������������
��3���ڶ���ʱ���Ӳ���������Һ���ƫ������c=$\frac{n}{V}$������������
��4����ͬ�����������������������أ������������ʵ����ࣻ
II����1��������������ܽ��Һ����Ҫ�÷�Һ����
��2�����뽺������Һ��Ӧѡ��������
��� I����1������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����480mL��ҺӦѡ��500mL����ƿ������ȱ�ٵ�������500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��2������0.1mol/L��KOH��Һ480mL��Ӧѡ��500mL����ƿ��ʵ������500mL��Һ����Ҫ���ʵ�����Ϊ��0.5L��0.1mol/L��56g/mol=2.8g��
�ʴ�Ϊ��2.8��
��3���ڶ���ʱ���Ӳ���������Һ���ƫ������c=$\frac{n}{V}$��֪����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ������
��4����ͬ�����������������������أ������������ʵ����࣬�������õĹ���KOH�л���NaOH�����������Һ��c��OH-��Ũ�ȣ�0.1mol/L��
�ʴ�Ϊ������
II����1�����Ȼ�̼��ˮ����ܽ⣬�ܶȲ�ͬ���ֲ㣬Ӧѡ���Һ���룻
��ѡ��D��
��2������ˮ��ҺΪ���岻������Ĥ��NaCl��Һ�ܹ�����Ĥ��������������������ߣ�
��ѡ��E��
���� ���⿼����һ�����ʵ���Ũ����Һ�����Ƽ����ʷ��뷽����ѡ����ȷ����ԭ�����������裬��ȷ���������ķ��뷽����ʹ�������ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����MnO2��H2SO4���������� | B�� | �����ڸ������п�ѭ��ʹ�� | ||
| C�� | ��������16gSʱת��1mol���� | D�� | ����MnSO4����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
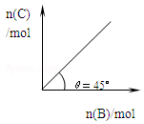 ��ʢ������A������ɱ���ܱ������У�����ѹǿһ��������B��������Ӧ��A��s��+2B��g��?4C��g��+D��g������H��0����һ���¶ȡ�ѹǿ�´ﵽƽ�⣮ƽ��ʱC�����ʵ���������B�����ʵ����ı仯��ϵ��ͼ������˵����ȷ����
��ʢ������A������ɱ���ܱ������У�����ѹǿһ��������B��������Ӧ��A��s��+2B��g��?4C��g��+D��g������H��0����һ���¶ȡ�ѹǿ�´ﵽƽ�⣮ƽ��ʱC�����ʵ���������B�����ʵ����ı仯��ϵ��ͼ������˵����ȷ����| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��ͼ��ʾװ�ÿ�������ȡ�۲�Fe��OH��2�ڿ����б�����ʱ��ɫ�ı仯��ʵ��ʱ����ʹ����м��4mol•L-1��������Һ�������Լ���ѡ����д���пհף�
��ͼ��ʾװ�ÿ�������ȡ�۲�Fe��OH��2�ڿ����б�����ʱ��ɫ�ı仯��ʵ��ʱ����ʹ����м��4mol•L-1��������Һ�������Լ���ѡ����д���пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ڢܢݢ� | C�� | �ۢܢ� | D�� | �٢ۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 46g�Ҵ��д��ڵĹ��ۼ�����Ϊ7NA | |
| B�� | 1L 0.1mol•L-1��NaHCO3-��Һ��HCO3-������Ϊ0.1NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | 235g�˻�${\;}_{92}^{235}$U�����ѱ䷴Ӧ��${\;}_{92}^{235}$U+${\;}_{0}^{1}$n$\stackrel{�ѱ�}{��}$${\;}_{38}^{90}$Sr+${\;}_{54}^{136}$U+10${\;}_{0}^{1}$n�����������ӣ�${\;}_{0}^{1}$n����Ϊ1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ | B�� | ʳ��ˮ | ||
| C�� | ����Ǧ���ؽ����Σ���Һ | D�� | Ũ���������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
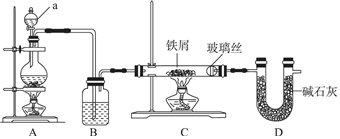
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com