
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�
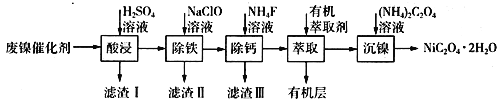
(1)��д��һ������ߡ���������ʵĴ�ʩ��________________________������I�ijɷ���____________(�ѧʽ)��
(2)����ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��
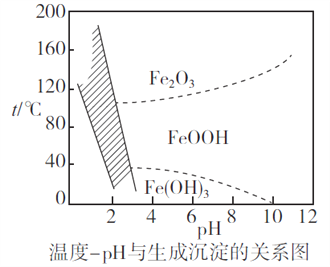
���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ_________________________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12](ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
(3)��֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_________mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
(4)�����л���ȡ����������________________________��
(5)ij��ѧ�����Լ��Ļ�ѧʽΪMxNi(SO4)y(MΪ+1�������ӣ�NiΪ+2�ۣ�x��y��Ϊ������)��Ϊ�ⶨ�ö����Լ�����ɣ���������ʵ�飺
I������28.7g�����Լ�������100 mL��ҺA��
��ȷ��ȡ10.00 mL��ҺA����0.40 mol��L-1��EDTA(Na2H2Y)����Һ�ζ����е�Ni2+(���ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+)������EDTA����Һ25.00mL��
����ȡ10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g��
������100 mL�����Լ�ʱ����Ҫ��������ҩ�ס�������ƽ�����������ձ�����Ͳ����ͷ�ι��⣬����Ҫ________________________��
�ڸö����Լ��Ļ�ѧʽΪ________________________________��
���𰸡� �ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ����� SiO2��CaSO4 FeOOH 2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+ 6.6��10-2 ��ȥ��Һ�е�Zn2+ 100mL����ƿ (NH4)2Ni(SO4)2
�����������������(1)����Ӱ�췴Ӧ���ʵ����ط�����ߡ���������ʵĴ�ʩ������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ��(2)��������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ�����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12������(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() ��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������
��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ������Ҫ��������������Ni2++H2Y2-=NiY2-+2H+����Ni2+�����ʵ���������![]() �ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
�ɼ�����Է�������������10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g���ɼ���SO42-�����ʵ���������n(Ni2+)��n(SO42-)����yֵ�����ݻ��ϼ۴����͵��������xֵ����������Է�����������M�����ԭ��������
������(1)����Ӱ�췴Ӧ���ʵ����أ������¶ȡ��ѷ����������顢�ʵ��������Ũ�Ȼ���������������ߡ����������������������SiO2�������Ӧ��CaO�����ᷴӦ�IJ���CaSO4����ˮ����������I�ijɷ���SiO2��CaSO4��(2) �ٸ�������II�ijɷ����¶ȡ�pH�Ĺ�ϵͼ����֪�����¶�40�桢pH=8ʱ������II����Ҫ�ɷ���FeOOH����Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ���+3����֪ClO-��Fe2+����ΪFe3+��ͬʱ����Na2Fe6(SO4)4(OH)12��������Ӧ�����ӷ���ʽ��2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��(3)���ݷ���ʽCa2++2F-= CaF2��������Ca2+����0.002mol NH4F ������Ksp(CaF2)=5.29��10-9������Ca2+����Һ��c(F-)=![]() �������c(NH4F)=c mol��L-1����
�������c(NH4F)=c mol��L-1����![]() =
=![]() ��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L
��c=6.6��10-2��(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��(5)�ٸ�������100mLһ�����ʵ���Ũ����Һ��Ҫ100mL����ƿ��������Ni2++H2Y2-=NiY2-+2H+��n(Ni2+)=0.025L![]() 0.4 mol��L-1=0.01mol ������
0.4 mol��L-1=0.01mol ������ ![]() ��MxNi(SO4)y����Է�������=
��MxNi(SO4)y����Է�������=![]() �� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96
�� 10.00 mL��ҺA������������BaCl2��Һ���õ����ᱵ4.66g������SO42-�����ʵ���0.02mol������n(Ni2+)��n(SO42-)=1��y����y=2�����ݻ��ϼ۴����͵�����,x=2���� M�����ԭ��������a����2a+59+96![]() =287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��
=287��a=18������M��NH4+���ö����Լ��Ļ�ѧʽΪ(NH4)2Ni(SO4)2��


 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������Ҫ������NaCl��Һ�����ֱ�ֻ�л���Na2SO4��NaHCO3��NaCl��ijѧ���������ͼ��ʾ������ȡ������NaCl��Һ��
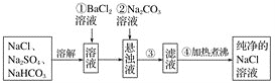
����˷�����ȷ����ô��
��1��������Ϊʲô�������ᱵ��Һ����������___________________________________��
��2�����в����ٺ�����ж�SO![]() �ѳ�����������___________________________��
�ѳ�����������___________________________��
��3�������ڵ�Ŀ����________��Ϊʲô���ȹ��˺��̼������Һ��������________________________________________________________________________��
��4���������õ��IJ����������ձ����������⣬����Ҫ____________________��
��5�������ܵ�Ŀ����______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڽ���Pt��Cu��ҿ��Ir���Ĵ������£��ܱ������е�H2�ɸ�Чת��������Һ�е���̬����NO3���Դﵽ������Ⱦ��Ŀ�ġ��乤��ԭ����ʾ��ͼ������

����˵������ȷ����
A. Ir�ı��淢����Ӧ��H2 + N2O == N2 + H2O
B. ��������ϵĸ�����Ӧ��H2��2e == 2H+
C. �����������ֻ�е�ԭ��ͭ��Ҳ������������Ⱦ��
D. ����������ϵ�Pt�������࣬�����ڽ�����Һ�еĺ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ������ȡSO2����֤SO2��ijЩ���ʵ�װ�ã���ش�
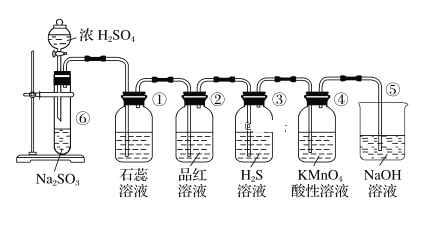
(1)�ڢ��з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
(2)���е�ʵ������Ϊʯ����Һ____________����ʵ��֤��SO2����____________�����ʡ�
(3)���е�Ʒ����Һ________,֤��SO2����____________�ԡ���
(4)���е�ʵ��������_______________________��֤��SO2��____________�ԡ�
(5)���е�ʵ��������______________________��֤��SO2��_____________�ԡ�
(6)��װ�õ�������___________________________���÷�Ӧ�����ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ȥ�����е�Ca2+. Mg2+. SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����
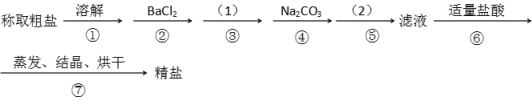
��1���ڢܲ��У�д����Ӧ�����ӷ���ʽ���������Һ��Ca2+����Ҫ������ʽΪCaCl2��_______________ ��____________��
��2��ʵ�鷽���ģ�1����Ӧʹ�ó����Լ��Ļ�ѧʽ__________�����������ӷ���ʽ��__________����ʵ�鷽���ģ�2���еIJ���������_______��
��3����ʵ����Ʒ����Ż��ĽǶȷ�������ںܿ͢ɷ�ߵ�____________����ǡ������������˵�����ɡ�___________________________________________��
��4���ж�BaCl2�ѹ����ķ�����_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ѽ��仯�����ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á�
(1)��̬��ԭ�ӵļ۵����Ų�ʽΪ_____________������ͬ���ڵ�Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ����____________�֡�
(2)�ѱȸ��ᡢ����Ӳ����һ�����˵Ľṹ���ϣ��ѵ�Ӳ�ȱ������ԭ����_________��
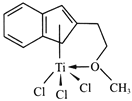
(3)��Ũ��TiCl3��������Һ�м������ѣ���ͨ��HCl�����ͣ��ɵõ���λ��Ϊ6�����ΪTiCl3��6H2O����ɫ���壬�þ�����������������ʵ���֮��Ϊ1��5�����������ӵĻ�ѧʽΪ___________��
(4)����Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ľۺϣ���ṹ����ͼ��ʾ��
�����M��Ԫ���У��縺��������_________(������)��
��M��̼ԭ�ӵ��ӻ���ʽΪ____________��
��M���________(�����)��
a���м� b���Ҽ� c�����Ӽ� d����λ��
(5)���ʯ(TiO2)�Ǻ��ѵ���Ҫ����֮һ���侧���ṹ(��������ͬλ�õ�ԭ����ͬ)��ͼ��ʾ��

��A��B��C��D 4������������ԭ����________(�����)��
����A��B��C��ԭ������ֱ�ΪA(0��0��0)��B(0.69a��0.69a��c)��C(a��a��c)����D��ԭ������ΪD(0.19a��____��___)���������ļ���d=______(�ô���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����48mL0.1mol/LHNO3��Һ�м���12mL0.4mol/LKOH��Һʱ�����õ�����Һ��
A.������ B.ǿ���� C.���� D.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҽ���NaCl��Һ����ʱ�������²������̣�����ȷ�IJ���˳����(�� )
�ٷ��þƾ��ƣ� �ڹ̶���Ȧλ�ã� �۷��������ܼ��Ƚ��裻 ��ֹͣ���ȣ���������
A.�ڢۢܢݢ�B.�٢ڢۢܢ�C.�ڢۢ٢ܢ�D.�ڢ٢ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ��
A. ��úת��Ϊ�������ȼ��B. ��úʱͨ���������
C. ��ֹʹ��ú̿D. ��ȼú�м���NaOH
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com