
£Ø10·Ö£©ŌŚŹµŃéŹŅ֊ѧɜÓĆĻĀ×óĶ¼×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£
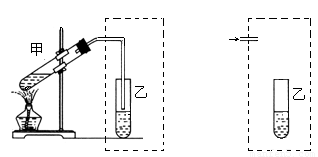
Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ“óŹŌ¹ÜÖŠ¼ÓČėÅØĮņĖį3mL”¢±ł“×Ėį3mL£Ø3 g£©”¢ŅŅ“¼4mL£Ø2.7 g£©£¬¼ÓČėŹŌ¼ĮµÄÕżČ·²Ł×÷ŹĒ__________________________________”£
£Ø2£©×°ÖĆÖŠĶØÕōĘūµÄµ¼¹ÜÖ»Äܲ嵽±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻ·½£¬²»²åČėČÜŅŗÖŠ£¬×÷ÓĆŹĒ__________£¬³¤µ¼¹ÜµÄ×÷ÓĆŹĒ_______________”£ŹŌÉč¼ĘĮķŅ»øö×°ÖĆŅ²ÄÜŹµĻÖÉĻŹöĮ½øö×÷ÓĆ£¬ŌŚŠéĻß²æ·Ö»³öøĆ×°ÖĆ¼ņĶ¼”£
£Ø3£©ŹŌ¹ÜŅŅÖŠµÄĻÖĻóŹĒ_______ £¬ÓÉ“ĖæÉÖŖŅŅĖįŅŅõ„µÄĪļĄķŠŌÖŹÓŠ_________________ ”£
£Ø4£©³ä·Ö·“Ó¦ŗó£¬ÖʵĆŅŅĖįŅŅõ„µÄÖŹĮæ_______________g”£
£Ø5£©¹¤ŅµÓĆŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·ÖĪŖŌĮĻÖĘČ”ŅŅĖįŅŅõ„£¬¾¹żµÄ·“Ó¦ĄąŠĶ£Ø°“·“Ó¦Ė³Šņ£©ŹĒ_________________________”£
CH3COOH£«CH3CH2OH 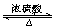 CH3COOC2H5£«H2O””
CH3COOC2H5£«H2O””
£Ø1£©ŌŚŹŌ¹ÜÖŠ¼Ó4mLŅŅ“¼£¬Č»ŗó±ßŅ”¶ÆŹŌ¹Ü±ßĀżĀż¼ÓČė3mLÅØĮņĖįŗĶ3mL ŅŅĖį”££ØÖ»ŅŖŗó¼ÓÅØĮņĖį£»±ßŅ”¶ÆŹŌ¹Ü±ßĀżĀż¼ÓŹŌ¼Į¾łæÉ£©
£Ø2£©·ĄÖ¹µ¹Īü µ¼Ęų”¢ĄäÄż
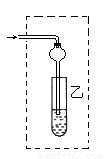
£Ø3£©±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻÓŠĶøĆ÷µÄÓĶדŅŗĢå²śÉś£¬æÉĪŵ½ĻćĪ¶”£
ŅŅĖįŅŅõ„²»ČÜÓŚĖ®£»ĆܶȱČĖ®Š”£»Ņ×»Ó·¢£Ø»ņ·ŠµćµĶ£©”£
£Ø4£©Š”ÓŚ4.4g
£Ø5£©¼Ó³É·“Ó¦”¢Ńõ»Æ·“Ó¦”¢õ„»Æ·“Ó¦
”¾½āĪö”æŹµŃéŹŅÖĘČ”ŅŅĖįŅŅõ„ÓĆŅŅĖįŗĶŅŅ“¼×÷ÓĆ£¬·½³ĢŹ½ĪŖCH3COOH£«CH3CH2OH 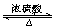 CH3COOC2H5£«H2O””
CH3COOC2H5£«H2O””
£Ø1£©ÅØĮņĖįČÜÓŚĖ®·Å³ö“óĮæµÄČČ£¬ĒŅĆܶȓóÓŚĖ®µÄ£¬ĖłŅŌÓ¦øĆŹĒŌŚŹŌ¹ÜÖŠ¼Ó4mLŅŅ“¼£¬Č»ŗó±ßŅ”¶ÆŹŌ¹Ü±ßĀżĀż¼ÓČė3mLÅØĮņĖįŗĶ3mL ŅŅĖį”£
£Ø2£©»Ó·¢³öĄ“µÄŅŅĖįŗĶŅŅ“¼¶¼ŹĒŗĶĖ®»„Čܵģ¬Čē¹ūÖ±½Ó²åČėČÜŅŗÖŠ£¬Ņ×µ¹Īü”£³¤µ¼¹ÜÄÜĘšµ¼ĘųŗĶĄäÄż×÷ÓĆ”£ŅŖĘšŅŌÉĻµÄ×÷ÓĆ£¬æÉÓĆŹśÖ±µÄøÉŌļ¹Ü¼“æÉ£¬ČēĶ¼ĖłŹ¾£Ø¼ū“š°ø£©”£
£Ø3£©ŅŅĖįŅŅõ„²»ČÜÓŚĖ®£¬ĖłŅŌŌŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻÓŠĶøĆ÷µÄÓĶדŅŗĢå²śÉś£¬ĒŅæÉĪŵ½ĻćĪ¶”£
£Ø4£©øł¾ŻĖłøųŹż¾ŻæŖŹ¼£¬ĄķĀŪÉĻÉś³ÉŅŅĖįŅŅõ„Ó¦øĆŹĒ3g£«2.3g£0.9g£½4.4g£¬µ«·“Ó¦µ«æÉÄę·“Ó¦£¬ĖłŅŌŹµ¼ŹÉś³ÉµÄŅŅĖįŅŅõ„Š”ÓŚ4.4g”£
£Ø5£©ĮŃ½āĘųÖŠŗ¬ÓŠŅŅĻ©£¬ŅŅĻ©ŗĶĖ®¼Ó³É¼“µĆµ½ŅŅ“¼£¬ŅŅ“¼Ńõ»Æ¼“µĆµ½ŅŅĖį£¬ŅŅĖįŗĶŅŅ“¼õ„»Æ¼“Éś³ÉŅŅĖįŅŅõ„”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
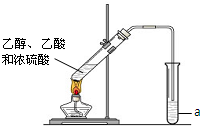 ŗģ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ĘäŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖ¾ßÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅæÉŅŌÓĆÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£®Ēė»Ų“š£ŗ
ŗģ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ĘäŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖ¾ßÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅæÉŅŌÓĆÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğĢģ½ņŹŠĢģ½ņŅ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©ŌŚŹµŃéŹŅ֊ѧɜÓĆĻĀ×óĶ¼×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£
Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ“óŹŌ¹ÜÖŠ¼ÓČėÅØĮņĖį3mL”¢±ł“×Ėį3mL£Ø3 g£©”¢ŅŅ“¼4mL£Ø2.7 g£©£¬¼ÓČėŹŌ¼ĮµÄÕżČ·²Ł×÷ŹĒ__________________________________”£
£Ø2£©×°ÖĆÖŠĶØÕōĘūµÄµ¼¹ÜÖ»Äܲ嵽±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻ·½£¬²»²åČėČÜŅŗÖŠ£¬×÷ÓĆŹĒ__________£¬³¤µ¼¹ÜµÄ×÷ÓĆŹĒ_______________”£ŹŌÉč¼ĘĮķŅ»øö×°ÖĆŅ²ÄÜŹµĻÖÉĻŹöĮ½øö×÷ÓĆ£¬ŌŚŠéĻß²æ·Ö»³öøĆ×°ÖĆ¼ņĶ¼”£
£Ø3£©ŹŌ¹ÜŅŅÖŠµÄĻÖĻóŹĒ_______ £¬ÓÉ“ĖæÉÖŖŅŅĖįŅŅõ„µÄĪļĄķŠŌÖŹÓŠ_________________ ”£
£Ø4£©³ä·Ö·“Ó¦ŗó£¬ÖʵĆŅŅĖįŅŅõ„µÄÖŹĮæ_______________g”£
£Ø5£©¹¤ŅµÓĆŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·ÖĪŖŌĮĻÖĘČ”ŅŅĖįŅŅõ„£¬¾¹żµÄ·“Ó¦ĄąŠĶ£Ø°“·“Ó¦Ė³Šņ£©ŹĒ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŹµŃéŹŅ֊ѧɜÓĆĻĀ×óĶ¼×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£
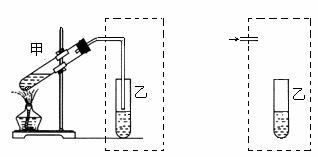 Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________”£
Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ“óŹŌ¹ÜÖŠ¼ÓČėÅØĮņĖį3mL”¢±ł“×Ėį3mL£Ø3 g£©”¢ŅŅ“¼4mL£Ø2.7 g£©£¬¼ÓČėŹŌ¼ĮµÄÕżČ·²Ł×÷ŹĒ__________________________________”£
£Ø2£©×°ÖĆÖŠĶØÕōĘūµÄµ¼¹ÜÖ»Äܲ嵽±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻ·½£¬²»²åČėČÜŅŗÖŠ£¬×÷ÓĆŹĒ__________£¬³¤µ¼¹ÜµÄ×÷ÓĆŹĒ_______________”£ŹŌÉč¼ĘĮķŅ»øö×°ÖĆŅ²ÄÜŹµĻÖÉĻŹöĮ½øö×÷ÓĆ£¬ŌŚŠéĻß²æ·Ö»³öøĆ×°ÖĆ¼ņĶ¼”£
£Ø3£©ŹŌ¹ÜŅŅÖŠµÄĻÖĻóŹĒ_______________ £¬ÓÉ“ĖæÉÖŖŅŅĖįŅŅõ„µÄĪļĄķŠŌÖŹÓŠ_________________ ”£
£Ø4£©³ä·Ö·“Ó¦ŗó£¬ÖʵĆŅŅĖįŅŅõ„µÄÖŹĮæ_______________g”£
£Ø5£©¹¤ŅµÓĆŹÆÓĶĮŃ½āĘųµÄÖ÷ŅŖ³É·ÖĪŖŌĮĻÖĘČ”ŅŅĖįŅŅõ„£¬¾¹żµÄ·“Ó¦ĄąŠĶ£Ø°“·“Ó¦Ė³Šņ£©ŹĒ_________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com