
������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ��������Ź㷺��Ӧ�á�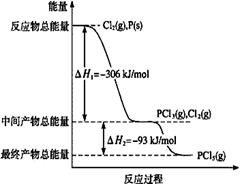
��1������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
��ش����⣺
��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ�� ��
��P��Cl2��������Ӧ����1 mol PCl5�ġ�H3�� ��
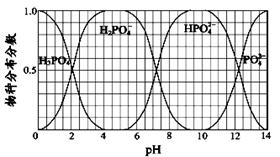
��2��PCl5�ֽ��PCl3��Cl2�ķ�Ӧ�ǿ��淴Ӧ��T��ʱ����2.0 L�����ܱ������г���1.0 mol PCl5������250 s�ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
| t / s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3) / mol | 0 | 0��16 | 0��19 | 0��20 | 0��20 |
��1����PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��2�֣�����ʽ1�֣���H�ı�ʾ1�֣���ѧʽ��״̬����0�֣�+���ʱ���ֵ����λ��©�Ͽ�1�֣��������÷�����ʾ���ʱ���ƥ��Ҳ���֣�
�ڣ�399 kJ/mol��2�֣���λ��©��1�֣�
��2����1.5��10-4mol/(L��s) ��0.00015 mol/(L��s)��2�֣���λ��©��1�֣�
��2.5��10-2 mol/L��0.025mol/L
��3����9��10.5��2�֣����ڴ�����������ڵ�ijһ�㣩
c(H2PO4��)��c(HPO42��) ��2�֣�
��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42��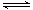 H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
H++PO43����1�֣�������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԣ�1�֣��������������֣�
���������������1����������ر�ע��ͼ������ת�����ʱ�������1mol��������ݣ����PCl5�ֽ��PCl3��Cl2�����ȷ�Ӧ�����Ȼ�ѧ����ʽΪ��PCl5(g)=PCl3(g)+Cl2(g)��H����93kJ/mol��
�ڼ���P��Cl2��������Ӧ����1 mol PCl5 ���ʱ䣬���ݸ�˹���ɷ�Ӧ���ʱ�������أ�ֻ�轫ͼ���������ֵ��ʱ���Ӽ��ɣ����ԡ�H3����399 kJ/mol��
��2���ټ��㷴Ӧ��50��150s �ڵ���PCl3��ʾ�ķ�Ӧ���ʣ�ֻ������ݴ��빫ʽ�������v(PCl3)����C/��t=(0��19-0��16)mol/(2L��100s)= 1.5��10-4mol/(L��s)��
�� PCl5(g) = PCl3(g)+Cl2(g)
��ʼŨ�ȣ�mol/L��  =0.50 0 0
=0.50 0 0
ת��Ũ�ȣ�mol/L�� 0.10 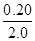 =0.10 0.10
=0.10 0.10
ƽ��Ũ�ȣ�mol/L�� 0.40 0.10 0.10 ��1�֣�
K�� 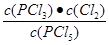 =
=  �� 2.5��10-2 mol/L��0.025mol/L
�� 2.5��10-2 mol/L��0.025mol/L
��3������Ҫѧ�������ͼ����ͼ��ʾ����H3PO4�벻�ϼ����NaOH��Ӧ��������������������ʹ��ҺpHֵ��������ʱ����Һ�и��������ֵİٷֱȡ�ÿ������������һ����PH��Χ�ڶ��аٷֺ�������ͼ�С��������������߽��沿�ֱ�ʾ������2�ֺ�������ͬʱ���ڵ������
��Ҫ��ýд�����Na2HPO4����ͨ��ͼ���ҵ�PHֵ��9~10.5�ķ�Χ�ڣ�HPO42- �İٷֺ����ӽ��ٷְ٣����Կ��Ƶ�PHֻҪ�������Χ�ڣ������Ի�ýϴ�����Na2HPO4 ��
��Na2HPO4��������ʽ�Σ�HPO42-���ڵ����ˮ������ƽ�⣬������Һ�ʼ��ԣ����Կ���ˮ��ǿ�ڵ��롣���Ǹ�����Һ�����Ȼ��ƺ���Һ�����ԣ���˿����Ʋ������Ȼ���һ���ı��˵���ƽ�⣬ʹ֮���ϲ����������ӣ�ʹ��Һ�����ԡ����Խ�ϱ������ɡ�������Ȼ�糣��������ˮ����������Ca3(PO4)2����ʽ���ڡ������Խ���ƽ����ƶ�Ϊ��Na2HPO4��Һ�д��ڵ���ƽ�⣬HPO42�� H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
H++PO43��������CaCl2��Һ��Ca2����PO43���������Ca3(PO4)2��������ʹNa2HPO4����ƽ�������ƶ���H+Ũ��������Һ�����ԡ�
���㣺���⿼����Ƿ�Ӧԭ����֪ʶ�������ڻ�ѧ�뷴Ӧ�����Ĺ�ϵ����Ӧ���ʺ�ƽ�ⳣ�����㡢ƽ���ƶ�ԭ�����͵ȷ���Ŀ��顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ѧ���õ�һ�����ᡣ
��1��ȡ0.10mol CH3COOH��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ��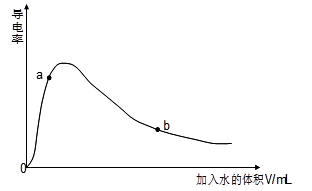
�ٿ�ʼʱ������Ϊ0˵���� ��
�� �Ƚ�a��b���������ʣ����������<������=������n(H+)��a b��c(CH3COO-)��a b����ȫ�к�ʱ����NaOH�����ʵ�����a b��
����b��ʱ����Һ��c(CH3COOH)=0.10mol/L��c(H+)=1.3��10-3mol/L�����ʱc(CH3COO-)ԼΪ mol/L������b��ʱ����ĵ���ƽ�ⳣ����д��������� ��
��2����֪��H+(aq) + OH-(aq) = H2O(l) ��H1="-57.3" kJ/mol
CH3COOH(aq)  H+(aq) +CH3COO-(aq) ��H2="+1.3" kJ/mol
H+(aq) +CH3COO-(aq) ��H2="+1.3" kJ/mol
д��ϡ������ϡ�ռ���Һ��Ӧ���Ȼ�ѧ����ʽ�� ��
��3�������£�ȡŨ�Ⱦ�Ϊ0.10mol/L�Ĵ������������Һ�������Ϻ����pH<6��д�������Һ�е������غ��ϵʽ �����г����е�����Ũ�ȴ�С˳���ɴ�С�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ�ֿ�����������ȼ�ϣ���;�㷺���о������þ��й���ǰ����
��1����֪�ڳ��³�ѹ�£���÷�Ӧ�ķ�Ӧ�����£�
�� 2CH3OH(l)+ 3O2(g)  2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
�� 2CO(g) +O2(g)  2CO2(g) ?H2=��566��0 kJ/mol
2CO2(g) ?H2=��566��0 kJ/mol
CH3OH����ȫȼ������CO����̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�������״��ķ�Ӧ���£�CO2(g) + 3H2(g)  CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
��ij�¶��£��ݻ���Ϊ1 L��A��B���������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݡ�����B�о�10 s��ﵽƽ�⡣�ﵽƽ��ʱ���й��������±���
| ���� | A | B |
| ��Ӧ��Ͷ���� | 1 mol CO2(g)��3 mol H2(g) | 1 mol CH3OH(g)��1 mol H2O(g) |
| ��Ӧ�����仯 | �ų���kJ���� | ����19��6 kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I�� C(s)��O2(g) = CO2(g)
;��II������ˮú���� C(s) + H2O(g) =" CO(g)" + H2(g)
ȼ��ˮú����2 CO(g) + O2(g) = 2CO2(g)��
2H2(g)+O2(g) =2H2O(g)
��֪����C(s)��O2(g)=CO2(g)����H1=��393.5 kJ��mol-1
��H2(g)+1/2O2(g)=H2O(g)����H2=��241.8kJ��mol-1
��CO(g)+ 1/2O2 (g) =CO2(g)����H3=��283.0kJ��mol-1
��ش��������⣺
��1��CO(g) + H2O(g) = H2(g) + CO2(g) �� ������ȷ�Ӧ�������ȷ�Ӧ����
��2�����ݸ�˹���ɣ�ú����̬ˮ����ˮú���ķ�Ӧ�ȡ�H= ��
��3����������;��������˵��������ǣ� ��
| A��;��II��ˮú��ʱ�����ܺģ���;��II����������ȡ |
| B����;��I��ȣ�;��II���Լ��ٶԻ�������Ⱦ |
| C����;��I��ȣ�;��II�������ú��ȼ��Ч�� |
| D����úת��Ϊˮú������ͨ���ܵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ú��Ϊȼ�Ͽ�ͨ����������;��:
;����C(s)+O2(g) CO2(g)����H1<0��
CO2(g)����H1<0��
;�������Ƴ�ˮú��:
C(s)+H2O(g) CO(g)+H2(g)����H2>0��
CO(g)+H2(g)����H2>0��
��ȼ��ˮú��:
2CO(g)+O2(g) 2CO2(g)����H3<0��
2CO2(g)����H3<0��
2H2(g)+O2(g) 2H2O(g)����H4<0��
2H2O(g)����H4<0��
��ش���������:
(1);����ų���������������������(����ڡ������ڡ���С�ڡ�);����ų���������
(2)��H1����H2����H3����H4����ѧ��ϵʽ������
(3)��֪:��C(s)+O2(g) CO2(g)����H1="-393.5" kJ��mol-1
CO2(g)����H1="-393.5" kJ��mol-1
��2CO(g)+O2(g) 2CO2(g)����H2="-566" kJ��mol-1
2CO2(g)����H2="-566" kJ��mol-1
��TiO2(s)+2Cl2(g) TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
TiCl4(s)+O2(g)����H3="+141" kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s) TiCl4(s)+2CO(g)�Ħ�H=����������
TiCl4(s)+2CO(g)�Ħ�H=����������
(4)��֪���и����Ȼ�ѧ����ʽ
��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
2Fe(s)+3CO2(g)����H1="-25" kJ��mol-1
��3Fe2O3(s)+CO(g) 2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
2Fe3O4(s)+CO2(g)����H2="-47" kJ��mol-1
��Fe3O4(s)+CO(g) 3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
3FeO(s)+CO2(g)����H3="+640" kJ��mol-1
��д��FeO(s)��CO(g)��ԭ��Fe��CO2(g)���Ȼ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
����NOx��һ�ַ��������ü������ԭNOx��
CH4(g)+4NO2(g)��4NO(g)+CO2(g)+2H2O(g) ��H1����574kJ��mol��1
CH4(g)+4NO(g)��2N2(g)+CO2(g)+2H2O(g) ��H2
CH4(g)+2NO2 (g)��N2(g) + CO2(g)+2H2O(g) ��H3����867kJ��mol��1
���H2�� ��
��ʯȼ�ϵ�ȼ�ա����������ʯ��ұ������������������в�����SO2�Ǵ�����SO2����Ҫ��Դ����1����úת��Ϊˮú���ǽ�úת��Ϊ�ྻȼ�ϵķ���֮һ����ӦΪ C(s) + H2O(g)�� CO(g) + H2(g)��
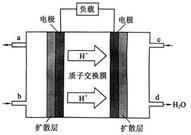
�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK�� �� 800��ʱ����1molCO��3mol H2O��1mol H2�����ݻ�Ϊ1L�������У�������Ӧ��CO(g) + H2O(g)  CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
a������� b�����¶� c��С������� d����CO2����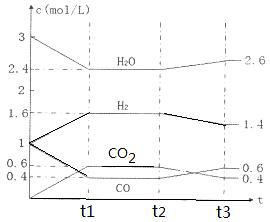
��2����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ�����Ƶ������������������£�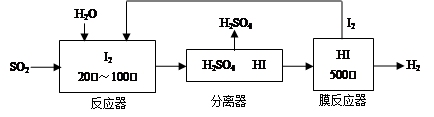
�������ӷ���ʽ��ʾ��Ӧ���з����ķ�Ӧ ��
���û�ѧƽ���ƶ���ԭ���������� HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H2��Ŀ���� ��
��������Դ�ǽ��������Ⱦ����Ч;��֮һ���״�ȼ�ϵ��(���DMFC)���ڽṹ������ת���ʸߡ��Ի�������Ⱦ,����Ϊ������Դ�����Ʒ��Խ��Խ�ܵ���ע��DMFC����ԭ����ͼ��ʾ��
ͨ��a����ĵ缫��ԭ��ص� ���������������
��缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��úת��Ϊˮú����ͨ����ѧ������úת��Ϊ�ྻȼ�ϵķ���֮һ��úת��Ϊˮú������Ҫ��ѧ��ӦΪ��C(s)��H2O(g) CO(g)��H2(g)��
CO(g)��H2(g)��
��C(g)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
��C(s)��O2(g)=CO2(g)����H1����393.5 kJ��mol��1
��H2(g)�� O2(g)=H2O(g)����H2����242.0 kJ��mol��1
O2(g)=H2O(g)����H2����242.0 kJ��mol��1
��CO(g)�� O2(g)=CO2(g)����H3����283.0 kJ��mol��1
O2(g)=CO2(g)����H3����283.0 kJ��mol��1
�������������Ϣ�ش��������⣺
(1)ú��һ�ֳɷָ��ӵĻ������г���̼����Ԫ���⣬���������������顢����Ԫ�ء����Թ���úȼ�ջᵼ�´�����Ⱦ��д��úȼ�ղ�����������Ⱦ����������������������ʯ��ʯ����ú�ۻ�ϣ�������Ч�ؼ���úȼ�չ����еĶ���������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________
(2)������֪�Ȼ�ѧ����ʽд����ú�Ʊ�ˮú�����Ȼ�ѧ����ʽ��____________________________��
(3)�����Ǽס�����λͬѧ�������Ȼ�ѧ����ʽ��úȼ�յ����⡣
��ͬѧ��1 mol CO��1 mol H2ȼ�շų�������֮�ʹ���1 mol����̿ȼ�շų�������������úȼ��ʱ��������ˮ������ʹúȼ�շų������������
��ͬѧ���������������������ѭ������ú̿ת��Ϊˮú������ȼ�շų���������ֱ��ȼ��ú̿�ų���������ͬ������ú̿ת��Ϊˮú�������������ģ���ú̿ת��Ϊˮú���ò���ʧ��
C(s)��H2O(g)��O2(g) CO2(g)��H2O(g)
CO2(g)��H2O(g)

CO(g)��O2(g)��H2(g) CO(g)��H2O(g)��
CO(g)��H2O(g)�� O2(g)
O2(g)
����������λͬѧ�����⣺
�ټ�ͬѧ��˵������������(����ȷ������ȷ��)��ԭ����______________________________________��
����ͬѧ��˵������������(����ȷ������ȷ��)��ԭ����_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺
(1)��Ԫ��ԭ�ӵ�L�������Ϊ________��
(2)�¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪����N2(g)��2O2(g)=N2O4(l)�� ��H1����19.5 kJ��mol��1
��N2H4(l)��O2(g)=N2(g)��2H2O(g)�� ��H2����534.2 kJ��mol��1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ________________________��
(3)��֪H2O(l)=H2O(g)����H3����44 kJ��mol��1�����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________��
(4)�¡�����ȼ�ϵ����һ�ּ��Ե�أ��õ�طŵ�ʱ�������ķ�ӦʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȩ��һ����Ҫ�Ļ�����Ʒ,�����ü״��������Ʊ�����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��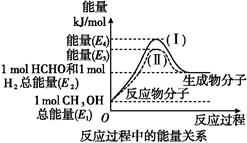
(1)�״�������ת��Ϊ��ȩ�ķ�Ӧ����������(����ȡ����ȡ�)��Ӧ��
(2)���̢�����̢�ķ�Ӧ���Ƿ���ͬ?��������,ԭ����
(3)д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ: ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com