
����Ŀ����1���ҹ��Ǽ��⼼���Ƚ��Ĺ��ң��챦ʯ��Al2O3�����������ڲ�������IJ��ϡ��������ӷ���ʽ��֤������һ�����������____________��___________��
��2��ȡ������������ijþ���Ͻ�ֱ����������ϡ���������������Һ�У������ı�״����H2����ֱ�Ϊ33.6L��22.4L��úϽ���þ���������ʵ���֮��Ϊ___________��
��3����һ��������þ���Ͻ��ܽ���500mL�����У���Ӧ�����Һ����μ���2mol/LNaOH��Һ�������������������Һ����Ĺ�ϵ��ͼ��ʾ������������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����ı仯���Բ��ƣ�_______________�������������������ֵ_____________g��
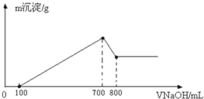
���𰸡�Al2O3+6H+=2Al3++3H2O Al2O3+2OH��=2AlO2��+H2O 3:4 2.8mol/L 33
��������
��1����������������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�����ų�������
��2������Mg+2HCl=MgCl2+ H2����2Al+6HCl=2AlCl3+3 H2����2Al+2NaOH+2H2O=2 NaAlO2+3 H2�����м��㣻
��3����ͼ���֪��������NaOH700mLʱ�����������ֵ����ʱ��Һ����Һ����ΪNaCl�����������ӡ��������غ��֪n��HCl��=n��NaCl��=n��NaOH�����ٸ���c=![]()
��������Ũ�ȣ�����NaOH800mLʱ������Al��OH��3+NaOH=NaAlO2+2H2O������������ȫ�ܽ⣬����NaOH100mL����ʱn��NaOH��=0.1L��2mol/L=0.2mol���Դ˿ɼ���Al��OH��3�����ʵ���������NaOH��Һ100mL��700mLʱ������Mg2++2OH-�TMg��OH��2����Al3++3OH-�TAl��OH��3������ϸý����ĵ�NaOH����n[Mg��OH��2]���ٸ���m=nM���㣮
��1����������������������ܺ�ǿ�ᷴӦ���ܺ�ǿ�Ӧ�����ӷ���ʽ�ֱ�Ϊ��Al2O3+6H+�T2Al3++3H2O��Al2O3+2OH-�T2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+6H+�T2Al3++3H2O��Al2O3+2OH-�T2AlO2-+H2O��
��2����þ�����ʵ�����xmol���������ʵ�����2ymol��þ���Ͻ���뵽����������Һʱ��ֻ����2Al+2NaOH+2H2O=2 NaAlO2+3 H2�������ݷ�Ӧ�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ���֪�������������ʵ�����3ymol��������֪3y=1mol���ɵ��������ʵ�����![]() ͬ���õ����ĺϽ������ᷴӦʱ�������ᷴӦ�ų�����������ڱ�״������Ӧ����22.4L��˵��û�����ᷴӦ���������������11.2L������£�����Mg+2HCl=MgCl2+ H2������֪þ�����ʵ�����0.5mol��þ���������ʵ���֮��Ϊ0.5mol��
ͬ���õ����ĺϽ������ᷴӦʱ�������ᷴӦ�ų�����������ڱ�״������Ӧ����22.4L��˵��û�����ᷴӦ���������������11.2L������£�����Mg+2HCl=MgCl2+ H2������֪þ�����ʵ�����0.5mol��þ���������ʵ���֮��Ϊ0.5mol��![]() =3:4��
=3:4��
�ʴ�Ϊ��3:4��
(3)��ͼ���֪��������NaOH700mLʱ,���������ֵ,��ʱ��Һ����Һ����ΪNaCl�����������ӡ��������غ��֪��n(HCl)=n(NaCl)=n(NaOH)=0.7L��2moL/L=1.4mol��
����������ʵ���Ũ��=1.4mol0.5L=2.8mol/L��
����������ʵ���Ũ��Ϊ2.8mol/L��
����NaOH800mLʱ������Al(OH)3+NaOH=NaAlO2+2H2O������������ȫ�ܽ⣬����NaOH100mL����ʱn(NaOH)=0.1L��2mol/L=0.2mol����֪Al(OH)3�����ʵ���=0.2mol������NaOH��Һ100mL700mLʱ������Mg2++2OH�TMg(OH)2����Al3++3OH�TAl(OH)3����������������������NaOHΪ0.2mol��3=0.6mol,������������þ����NaOHΪ(0.70.1)L��2mol/L0.6mol=0.6mol,��n[Mg(OH)2]=0.6mol2=0.3mol����������������=0.3mol��58g/mol+0.2mol��78g/mol=33g��
�𣺳������������Ϊ33g.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
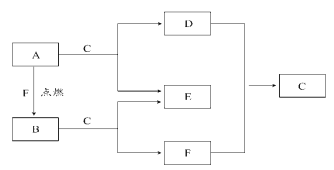
����Ŀ��A��D��F���dz���Ԫ����ɵĵ��ʣ�B�ǵ���ɫ���壬������C��Һ�塣
��ش��������⣺
��1��B��E�Ļ�ѧʽ��B__��E___��
��2��A��C��Ӧ�����ӷ���ʽΪ___��A��F����B�Ļ�ѧ����ʽΪ___��
��3������A����������ȷ����___(�����)��
��A�к�ǿ�Ļ�ԭ�� ��A����ɫ��Ӧ����ɫ
������A���Ա�������ˮ�� ��A�Ż�ʱӦ��ϸɳ����
��4����A��þ������0.3mol�ֱ����100mL1mol/L�������У�ͬ��ͬѹ�²������������֮����__��
��1��2��3 ��6��3��2 ��3��1��1 ��1��1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺
��1��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�ϣ�ͨ�����¹����Ʊ�ClO2��
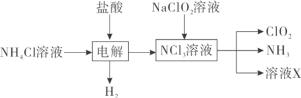
�ٵ��ʱ�����缫��ӦʽΪ__________________________��
�ڳ�ȥClO2�е�NH3��ѡ�õ��Լ���___________�����ţ���
a��ˮ b����ʯ�� c��Ũ���� d������ʳ��ˮ
��2������ͼװ�ÿ��Բⶨ�������ClO2�ĺ�����
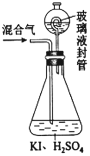
������ƿ�м��������ĵ⻯�أ���50mLˮ�ܽ���ټ���3mLϡ���
���ڲ���Һ��װ���м���ˮ��ʹҺ��û������Һ��ܵĹܿڣ�
��һ�����Ļ������ͨ����ƿ�����գ�
����������Һ��װ���е�ˮ������ƿ�У�
������0.1000mol��L-1��������Ʊ���Һ�ζ���ƿ�е���Һ��I2+2S2O32-��2I��+S4O62-����ָʾ����ʾ�յ�ʱ����ȥ20.00mL�����������Һ���ڴ˹����У�
����ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ______________________��
�ڲ���Һ��װ�õ�������______________________��
��V�м���ָʾ�����ζ����յ��������______________________��
�ܲ�û������ClO2������Ϊ______g��
��ijͬѧ��ij���̶ֿ�ģ�������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ�洦����ͼ��ʾ�Ŀ̶ȴ��������Һ������________������ţ���
a������23.60mL b������27.60mL c����23.60mL d������27.60mL
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϊ�ĸ����õĵ绯ѧװ�ã��������ǵ�������ȷ����
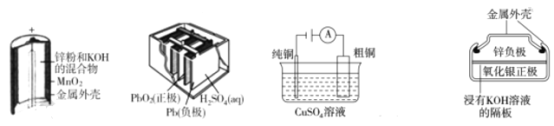
A.ͼ(a)�У�MnO2�������Ǵ���
B.ͼ(b)��ʾ��طŵ�����У��������������������
C.ͼ(c)��ʾװ�ù��������У��������Һ��Cu2+Ũ��ʼ�ղ���
D.ͼ(d)��ʾ��س������У�Ag2O������������ع��������л�ԭΪAg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ӹ����������仯��������������������ж����˾�����á�
��1���Ŵ��й��Ĵ���֮һ��ָ����������Ȼ��ʯ�Ƴɵģ�����Ҫ�ɷ���________��
A��Fe B��FeO C��Fe3O4 D��Fe2O3
��2�����ִ���ҵ�����У������� FeCl3 ��ʴͭ��ԭ������ӡˢ��·�壬д����ԭ���Ļ�ѧ����ʽ_________________________
��3��ʵ���������� FeSO4 ��ҺʱΪ�˷�ֹ FeSO4 ��Һ���ʣ����������м������ۣ���ԭ����_______________(�����ӷ���ʽ��ʾ) ��
��4�������������õ� 100mL 6mol/L FeSO4 ��Һ�������е���һ������ϡ���ᣬ�ش��������⣺
����ƽ�÷�Ӧ�����ӷ���ʽ����Fe2������NO3-����H�� = ��Fe3������NO������H2O___________
��Ҫ����÷�Ӧ�����Һ���Ƿ��� Fe2������ѡ�õ��Լ�Ϊ___________
A. ���� KMnO4 ��Һ B.KSCN ��Һ C. Cl2
�۾����飬������Ӧ�����Һ�в����� Fe2������÷�Ӧ�����в����� NO ���Ϊ����״���£�_____________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Ӧ�з�Ӧ�����������У�AsH3��H2SO4��KBrO3��K2SO4��H3AsO4��H2O��һ��δ֪����X��
��1����֪KBrO3�ڷ�Ӧ�еõ����ӣ�����_____�����������ԭ������Ӧ����÷�Ӧ�Ļ�ԭ����________��
��2����֪0.2molKBrO3�ڷ�Ӧ�еõ�1mol��������X����X�Ļ�ѧʽΪ____��
��3������������Ӧ����֪________(�����)��
a�������ԣ�KBrO3>H3AsO4
b�������ԣ�H3AsO4>HBrO3
c����ԭ�ԣ�AsH3>X
d����ԭ�ԣ�X>AsH3
��4�����������ͻ�ԭ���Ļ�ѧʽ������ƽ���ϵ���������з����У����������ת�Ƶķ������Ŀ��_____________
![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20 mLijŨ�ȵ�AlCl3��Һ�еμ�2 mol��L��1��NaOH��Һʱ���õ�Al(OH)3����������(g)�����μ�NaOH��Һ�����(mL)��ϵ��ͼ��ʾ���Իش��������⣺
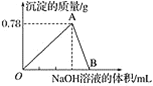
(1)ͼ��A���ʾ�ij�����__________(д��ѧʽ)�������ʵ���Ϊ____________��
(2)��Ӧ��A��ʱ����NaOH��Һ�����Ϊ______________��
(3)ͼ��B����Һ�е�������________________��
(4)AlCl3��Һ��Ũ��Ϊ______________��
(5)O�㵽B�㷴Ӧ�������ӷ���ʽ�ɱ�ʾΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ߴ�������̼��Ҫ����ҽѧ�о����ٴ���ϼ����ӹ�ҵ����̼���л��ﴼ��ȩ�������������й㷺���á�
I��(1)��ҵ����CO2��H2��һ�������·�Ӧ�ɺϳɶ����ѣ���֪��
2CO2(g)+6H2(g)��2CH3OH(g)+2H2O(g) ��H1����107.4kJ/mol
2CH3OH(g)��CH3OCH3(g)+H2O(g) ��H2����23.4kJ/mol
��2CO2(g)+6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H3��________kJ/mol
CH3OCH3(g)��3H2O(g) ��H3��________kJ/mol
(2)��һ�������½�CO2��H2����һ�̶��ݻ����ܱ������У������ֲ�ͬ�¶��·�����Ӧ��2CO2(g)+6H2(g)![]() CH3OCH3(g)+3H2O(g)�����CH3OCH3(g)�����ʵ�����ʱ��ı仯��ͼ��ʾ��
CH3OCH3(g)+3H2O(g)�����CH3OCH3(g)�����ʵ�����ʱ��ı仯��ͼ��ʾ��
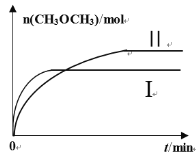
������I�����Ӧ��ƽ�ⳣ����С��ϵΪKI_______K��(�������=������)��
��һ���¶��£��������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����________(�����)��
a����������ܶȲ���
b�������Ѻ�ˮ�����ķ�Ӧ����֮�ȱ��ֲ���
c��v��(H2)=2v��(H2O)
d��2��C=O���ѵ�ͬʱ��3��H��O����
(3)�ϳ���CO��H2��һ���������ܷ������·�Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H����ijѹǿ�£��ϳɼ״��ķ�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO��ת������ͼ��ʾ��
CH3OH(g) ��H����ijѹǿ�£��ϳɼ״��ķ�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO��ת������ͼ��ʾ��
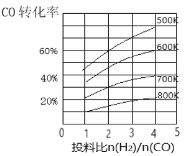
��600K�¶��£���1molCO��4molH2����2L���ܱ������У�5min��Ӧ�ﵽƽ��״̬����0��5min�ڵ�ƽ����Ӧ����v(H2)��___________��
����Ͷ�ϱȱ��ֲ��䣬�����¶ȣ��÷�Ӧƽ����_______�����ƶ�(�����Ӧ�����淴Ӧ��)��
�������ϳɼ״��Ĺ��������CO��ת���ʿɲ�ȡ�Ĵ�ʩ��______��(�о�һ�ּ���)��
���ø�Ĥ��ⷨ������Ũ����ȩ��ˮ��ԭ��Ϊ��
ʹ�ö��Ե缫��⣬��ȩ�ֱ�����������ת��Ϊ�Ҵ������ᣬ�ܷ�ӦΪ��2CH3CHO+H2O![]() CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ��Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̡�
CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ��Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̡�
�ٵ������У��������ֱ�����������Ҵ��⣬��������ɫ���壬������������ĵ缫��ӦΪ��______��
����ʵ�ʹ��մ��������У���������ȩ��ȥ���ʿɴ�80%�������������ֱ�ע��1m3��ȩ����Ϊ400mg/L�ķ�ˮ���ɵõ��Ҵ�________kg(����������2λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ��������Ҫ������������ʾ��

�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gH2O����
�¶� ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | - | - | - | - |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
��ʾ���¶ȸ���35��ʱNH4HCO3��ֽ⣬��ش�
��1�����в�����������ȷ����________��
A���¶ȿ�����30-35������Ϊ�¶�̫��NH4HCO3��ֽ⣬�¶�̫�ͷ�Ӧ����̫��
B������30min��Ŀ����ʹ��Ӧ��ֽ���
C�����˺����Һֻ��NH4Cl��NH4HCO3����
D��ϴȥ�����������ʿ���ѡ������ˮ
��2����Ӧ�¶ȿ�����30��35�棬Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ______________��
��3������ʱ�����˺���Ҫ�õ�NaHCO3�����ԭ����______________��
��4������NaHCO3�����װ��Ϊ________��
A.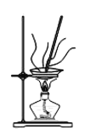 B.
B.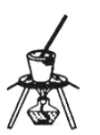 C.
C.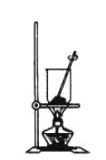
��5��ϴ��NaHCO3����IJ���______________��
��6���ⶨ�����Ʒ��NaHCO3�����ķ�����ȷ��ȡ������ƷWg������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc��mol��L-1����HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ(ָʾCO32-+H+=HCO3����Ӧ���յ�)����HCl��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ���ΪV2mL��д��������Ʒ��NaHCO3���������ļ���ʽ��______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com