
 ��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣
��1�����ݷ�ӦNa2SO3(��)��H2SO4(Ũ)��Na2SO4��SO2��H2O���Ʊ�SO2���塣 �������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��
�������м�ͼ���ڴ���ķ����л����Ʊ����ռ�SO2��ʵ��װ��(���Լ�)ʾ��ͼ��
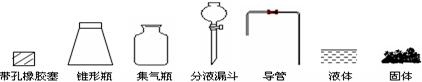
 ��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��
��ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� �� ��2����SO2����ֱ�ͨ��������Һ�У�
��2����SO2����ֱ�ͨ��������Һ�У� ��Ʒ����Һ�������� ��
��Ʒ����Һ�������� �� ����ˮ��Һ�������� ��
����ˮ��Һ�������� �� ��������Һ�������� ��
��������Һ�������� �� ��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ���������
��3����һС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⡣�����Ʋ���ܵ�ԭ��˵����Ӧ����֤���������Բ��������� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� �� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� �� ��ԭ�� ����֤���� ��
��ԭ�� ����֤���� ��
 �ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ�
�ڴ�Һ©���ϿڵĻ�����������Һ©���������������μ� ��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ�
��2������Һ��ɫ �� ��Һ��ɫ �� ��dz��ɫ��Һ������Һ����ǣ� ��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3�������
��3����Na2SO3����ȡ�����������Թ��У�����������ˮ�����Һ���ȵ�������ϡ���ᣬ�ٵ���BaCl2��Һ�а�ɫ�������ɣ���֤����Na2SO3������� ��1�����ݷ�Ӧԭ������ĿҪ���Ʊ�SO2���ռ������ǵ�SO2�ж��ȿ��Ի���װ��ͼ����2��SO2����ͨ��Ʒ����ҺƷ����ɫ��ͨ����ˮ����������ˮ����ԭ��Br-����ɫ��ͨ��Na2S��Һ����2S2-+SO2 +4H+
��1�����ݷ�Ӧԭ������ĿҪ���Ʊ�SO2���ռ������ǵ�SO2�ж��ȿ��Ի���װ��ͼ����2��SO2����ͨ��Ʒ����ҺƷ����ɫ��ͨ����ˮ����������ˮ����ԭ��Br-����ɫ��ͨ��Na2S��Һ����2S2-+SO2 +4H+ 3S��+2H2O����dz��ɫ���ǡ���3���������������⣬����ԭ�ֱ�Ϊ��Na2SO3���ʲ�����Na2SO4������HCl��BaCl2��֤�����õIJ���ŨH2SO4��������ŨH2SO4����ˮ�Ե�������֤��
3S��+2H2O����dz��ɫ���ǡ���3���������������⣬����ԭ�ֱ�Ϊ��Na2SO3���ʲ�����Na2SO4������HCl��BaCl2��֤�����õIJ���ŨH2SO4��������ŨH2SO4����ˮ�Ե�������֤��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȫ����FeS | B����Fe��FeS | C��FeS��S | D����Fe��FeS��S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeCl3��Һ | B��AlCl3��Һ | C��Na2S��Һ | D��AgNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
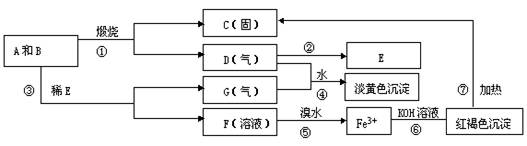
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2 | B��SO3 O2 | C��SO2 O2 | D��SO2 SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��С��0.45mol | B������0.45mol | C����0.45mol��0.90mol֮�� | D������0.90mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ����I | ������ | �ж� |
| A | ��ҵ����������ˮ����SO3 | SO3����ˮ��Ӧ | I�ԣ���ԣ��� |
| B | Cl2��SO2��Ϻ������Ư��ֽ�� | Cl2��SO2���нϺõ�Ư������ | I�ԣ�������� |
| C | �����ƾ���ǿ��ԭ�� | ��ѹ�ƵƷ�������ǿ�Ĺ� | I�ԣ���ԣ��� |
| D | ʯī���������صĵ缫 | ʯī�Ļ�ѧ�����ȶ��ҵ����Ժ� | I�ԣ���ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO+H2O?H2SO3 | B��SO2+2NaOH=Na2SO3+H2O | |||
C��2SO2+O2
| D��SO2+CaO=CaSO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com