
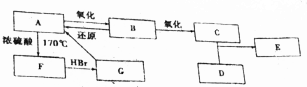
���� �л���A��B��C��D��E��F��G����F��G�⣬�����Ķ��dz��������ĺ��������A��������������C��C����Է�������Ϊ60�����֪AΪCH3CH2OH��A������BΪCH3CHO��B������CΪCH3COOH��D����Ĵ�����ΪCH3OH��E������C��D����������Ӧ��EΪCH3COOCH3��A��Ũ���������¼��ȵ�170���FΪCH2=CH2��F���� ���ⷢ���ӳɷ�Ӧ��GΪCH3CH2Br���ݴ˴��⣮
��� �⣺�л���A��B��C��D��E��F��G����F��G�⣬�����Ķ��dz��������ĺ��������A��������������C��C����Է�������Ϊ60�����֪AΪCH3CH2OH��A������BΪCH3CHO��B������CΪCH3COOH��D����Ĵ�����ΪCH3OH��E������C��D����������Ӧ��EΪCH3COOCH3��A��Ũ���������¼��ȵ�170���FΪCH2=CH2��F���� ���ⷢ���ӳɷ�Ӧ��GΪCH3CH2Br��
��1����������ķ�����֪��B�����ʽṹ��ʽΪ CH3CHO���ʴ�Ϊ��CH3CHO��
��2��AΪCH3CH2OH��A�Ĺ�������-OH���ʴ�Ϊ��-OH��
��3��GΪCH3CH2Br��CH3CH2Br�ڼ��������·���ˮ����Ҵ�����Ӧ�Ļ�ѧ����ʽ��CH3CH2Br+NaOH$��_{��}^{ˮ}$CH3CH2OH+NaBr����Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��CH3CH2Br+NaOH$��_{��}^{ˮ}$CH3CH2OH+NaBr��ȡ����Ӧ��
��4����һ���������£�C��D����������Ӧ����EΪ�����������Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3OH$��_{��}^{ŨH_{2}SO_{4}}$CH3COOCH3+H2O��������E��Ϊͬ���칹��������������л���ṹ��ʽΪHCOOCH2CH3��
�ʴ�Ϊ��CH3COOH+CH3OH$��_{��}^{ŨH_{2}SO_{4}}$CH3COOCH3+H2O��HCOOCH2CH3��
���� ���⿼���л����ƶϣ��漰ϩ��±����������ȩ������֮���ת����ϵ�ȣ��ѶȲ���ע�����֪ʶ���������գ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
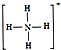 �����ף�P4���ĽṹʽΪ
�����ף�P4���ĽṹʽΪ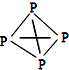 ���뻭��N4H44+�Ľṹʽ
���뻭��N4H44+�Ľṹʽ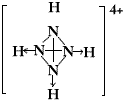 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ�����ӷ���ʽΪ��5Cl2+10 OH-�T7Cl-+2ClO-+ClO3-+5H2O | |
| B�� | �÷�Ӧ�У��������뻹ԭ�����ʵ���֮��Ϊ5��3 | |
| C�� | �������Լ���Һ�к���0.3mol��KOH | |
| D�� | ��Ӧ�����ɵ�ClO-�� ClO3- ����������һ�������¾��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���벹��������
���벹��������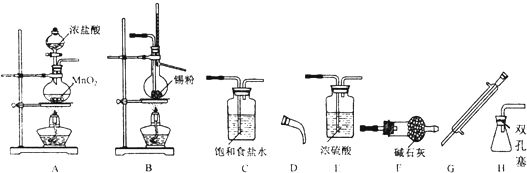
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��NH4+��NO3-��MnO4- | B�� | Na+��NO3?��SO42?��I? | ||
| C�� | K +��Fe3+��Cl-��Br- | D�� | Ba2+��Na+��OH-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
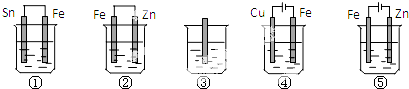
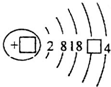
| A�� | �٢ڢۢܢ� | B�� | �ۢڢ٢ݢ� | C�� | �ݢ٢ۢڢ� | D�� | �ݢڢ٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ��-C2H5OH | B�� | ����-KAl��SO4��2 | C�� | �մ�-NaHCO3 | D�� | ��ʯ��-CaO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ø����pH��ֽ�ⶨNaClO��Һ��pH | |
| B�� | �����µ�ʯӢ�����н����ۻ��������ƹ����ʵ�� | |
| C�� | �÷�Һ©�������屽��ˮ�Ļ����ʱ���屽���¿ڷų���ˮ���Ͽڵ��� | |
| D�� | ��������������Ϊ10%������ͭ��Һ����ȷ��ȡ10 g����ͭ��������90 gˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com