
����ѧ�����ʽṹ�����ʡ�
�±���ʵ����Ԫ�����ڱ��IJ��ֱ߽磬�����ϱ߽粢δ��ʵ�߱����

������Ϣ�ش��������⣺
��1�����ڱ��б�Ga��������2�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ ��
��2��FeԪ��λ�����ڱ��� ������Fe��CO���γ������Fe(CO)5����Fe(CO)5�����Ļ��ϼ�Ϊ__________����֪��ԭ����Ŀ�͵�����������۵�����������ͬ������Ϊ�ȵ����壬�ȵ�����������ƵĽṹ��������CO���ӻ�Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ��������____��������_____���ѧʽ����CO�ĽṹʽΪ�� ��
��3����CH4��CO��CH3OH�У�̼ԭ�Ӳ�ȡsp3�ӻ��ķ���Ϊ��������������������
��4������VSEPR����Ԥ��ED4- ���ӵĿռ乹��Ϊ______________�͡�B��C��D��Eԭ��������γɵķ����У�����ԭ�Ӷ����������8�����ȶ��ṹ�ĵ���ʽΪ��__________________________________(д2��) ��
��5��B��D�γɵ��ȶ�������Ϊ___________���ӣ�����ԡ����Ǽ��ԡ��������̬Ϊ ________���塣
 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| I1/kJ?mol-1 | I2/kJ?mol-1 |
I3/kJ?mol-1 |
I4/kJ?mol-1 |
I5/kJ?mol-1 |
| 738 | 1451 | 7733 | 10540 | 13630 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ�
��2010?���϶�ģ������ѧ-���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
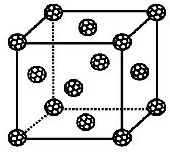 ����ѧ-���ʽṹ�����ʡ�
����ѧ-���ʽṹ�����ʡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
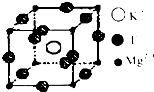 ����ѧ--���ʽṹ�����ʡ�
����ѧ--���ʽṹ�����ʡ�- 2 |
- 3 |
- 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com