
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������:

���Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ????????????????? ?????? ��
����ͼ��ʾ�����˲����е�һ��������?????? ????????? ????????? ���ж������г����Ƿ�ϴ�����õ��Լ���??????????? �����±���ʱ������ʢ�Ź��������������???? ?????????? ?????? ��

����25���£���Ũ�Ⱦ�Ϊ0��01 mol?L-1��MgCl2��AlCl3�����Һ����μ��백ˮ��������_______����(�ѧʽ)�����ɸó��������ӷ���ʽ_______?? ______________
����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��Ksp[Al(OH)3]=3��10 -34����
����ˮAlCl3(183������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ���

װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ������??? ????????????? �� F���Լ���������???? ?????? ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ???????????????? ?? ��
�ɽ�Mg��Cu��ɵ�3.92g�����Ͷ�����ϡ�����У���ַ�Ӧ������ȫ�ܽ�ʱ�ռ�����ԭ����NO����1.792L����״��������Ӧ�����Һ�м���4mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ���������γɳ���������Ϊ??????????????? g��
(1��? 2 Al(OH)3+Mg (OH)2 MgAl2O4 + 4H2O??
MgAl2O4 + 4H2O??
(2��©���¶˼���δ�����ձ��ڱ�??? AgNO3��Һ(�������ữ��AgNO3��Һ)������
(3��Al(OH)3??????? Al3++ 3NH3��H2O��Al(OH)3��+ 3NH4+
(4����ȥHCl������ˮ��������ʯ��(��NaOH��CaO�����)
(5��8.00
��������
 �����������1����MgCl2��AlCl3�Ļ����Һ�м�������İ�ˮ��������Ӧ��MgCl2+ 2NH3��H2O��Mg(OH)2��+ 2NH4Cl��AlCl3+ 3NH3��H2O��Al(OH)3��+ 3NH4Cl��Ȼ���Al(OH)3��Mg (OH)2�������˳�������ϴ�Ӹɾ���������������շ�����Ӧ2Al(OH)3+Mg (OH)2
�����������1����MgCl2��AlCl3�Ļ����Һ�м�������İ�ˮ��������Ӧ��MgCl2+ 2NH3��H2O��Mg(OH)2��+ 2NH4Cl��AlCl3+ 3NH3��H2O��Al(OH)3��+ 3NH4Cl��Ȼ���Al(OH)3��Mg (OH)2�������˳�������ϴ�Ӹɾ���������������շ�����Ӧ2Al(OH)3+Mg (OH)2 MgAl2O4 + 4H2O�����õ�Ҫ�Ʊ�MgAl2O4��������ͼ��ʾ�Ĺ��˲����еĴ�����©���¶˼���δ�����ձ��ڱڡ����ڳ����Ǵӽ����Ȼ����й��˳����ģ�����Ҫ�ж������г����Ƿ�ϴ���ķ��������������ữ��AgNO3��Һ�������Ƿ���Cl-���ɡ����±���ʱ��Ҫ��������ʢ�Ź������ʡ���Mg2+�γɳ�����Ҫ��
MgAl2O4 + 4H2O�����õ�Ҫ�Ʊ�MgAl2O4��������ͼ��ʾ�Ĺ��˲����еĴ�����©���¶˼���δ�����ձ��ڱڡ����ڳ����Ǵӽ����Ȼ����й��˳����ģ�����Ҫ�ж������г����Ƿ�ϴ���ķ��������������ữ��AgNO3��Һ�������Ƿ���Cl-���ɡ����±���ʱ��Ҫ��������ʢ�Ź������ʡ���Mg2+�γɳ�����Ҫ��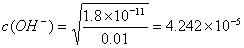 ����Al3+�γɳ�����Ҫ��
����Al3+�γɳ�����Ҫ�� <4.242��10-5����Ҫ��OH-��Ũ��ԽС��Խ�����γɳ�������������γɵ���Al(OH)3��������4��װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ�����dz�ȥ��������������HCl��ͬʱ��С�������ܽ⡣������ˮAlCl3(183������)����ʪ��������������������������F���Լ�������������ˮ��������ֹ��ˮAlCl3ˮ�⡣�ø����װ���ʯ��Ҳ����F��G�����á���Mg��Cu�����ᷢ����Ӧ��ΪMg2+��Al3+ʱʧȥ�ĵ��ӵ����ʵ�����HNO3�õ����ӵ����ʵ�����ȡ�������Ӧ�����Һ�м���NaOH��Һ��������ǡ����ȫ����ʱ���ӵ���������OH-�������������ʵ����͵���Mg2+��Al3+ʱʧȥ�ĵ��ӵ����ʵ��������ݵ�����ϵ�ɵá�n(NO)= 1.792L��22.4L/mol=0.08mol.����n(e-)=0.08mol��3=0.24mol. ���γɳ���������Ϊ3.92g��0.24mol��17g/mol=8.00g��
<4.242��10-5����Ҫ��OH-��Ũ��ԽС��Խ�����γɳ�������������γɵ���Al(OH)3��������4��װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ�����dz�ȥ��������������HCl��ͬʱ��С�������ܽ⡣������ˮAlCl3(183������)����ʪ��������������������������F���Լ�������������ˮ��������ֹ��ˮAlCl3ˮ�⡣�ø����װ���ʯ��Ҳ����F��G�����á���Mg��Cu�����ᷢ����Ӧ��ΪMg2+��Al3+ʱʧȥ�ĵ��ӵ����ʵ�����HNO3�õ����ӵ����ʵ�����ȡ�������Ӧ�����Һ�м���NaOH��Һ��������ǡ����ȫ����ʱ���ӵ���������OH-�������������ʵ����͵���Mg2+��Al3+ʱʧȥ�ĵ��ӵ����ʵ��������ݵ�����ϵ�ɵá�n(NO)= 1.792L��22.4L/mol=0.08mol.����n(e-)=0.08mol��3=0.24mol. ���γɳ���������Ϊ3.92g��0.24mol��17g/mol=8.00g��
���㣺�������ʵ��Ʊ��������ķ��롢������ѡ����ʹ�á��������ȡ�����ʵij�ȥ�������γɵ��Ⱥ�˳���غ㷨�ڻ�ѧ�����е�Ӧ�õ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿����ģ���ѧ�Ծ���B�������������� ���ͣ�ʵ����
[2012��ɽ����] ��11�֣�ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ�������£�
��1��ΪʹMg2����Al3��ͬʱ���ɳ�����Ӧ���������Ӧ���м���________(�A����B��)���ٵμ���һ��Ӧ�
��2������ͼ��ʾ�����˲����е�һ��������____________________________��
��3���ж������г����Ƿ�ϴ�����õ��Լ���________�����±���ʱ������ʢ�Ź��������������________��
��4����ˮAlCl3(183 ������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ��� ����
����
װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ������________��F���Լ���������_____________________________��
��һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ��ɫ��У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������:


��1���Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ????????????? ��
��2����ͼ��ʾ�����˲����е�һ��������????????????? ?? ��

�ж������г����Ƿ�ϴ�����õ��Լ���??????????? �����±���ʱ������ʢ�Ź�?? �������������??????????? ��
��3����25���£���Ũ�Ⱦ�Ϊ0��01 mol?L-1��MgCl2��AlCl3�����Һ����μ��백ˮ��������_________________����(�ѧʽ)�����ɸó��������ӷ���ʽ_____________________����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��Ksp[Al(OH)3]=3��10 -34����
��4����ˮAlCl3(183������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ���

װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ������????????? ��F���Լ���������???????? ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ??????????????????? ��
��5����Mg��Cu��ɵ�3.92g�����Ͷ�����ϡ�����У���ַ�Ӧ������ȫ�ܽ�ʱ�ռ�����ԭ����NO����1.792L����״��������Ӧ�����Һ�м���4mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ���������γɳ���������Ϊ??????????????????????? g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�춫����ʡ���н���Э����������Ͽ������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������:

(1)�Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ ��
(2)��ͼ��ʾ�����˲����е�һ�������� ��

�ж������г����Ƿ�ϴ�����õ��Լ��� �����±���ʱ������ʢ�Ź�������������� ��
(3)��25���£���Ũ�Ⱦ�Ϊ0��01 mol・L-1��MgCl2��AlCl3�����Һ����μ��백ˮ��������__________����(�ѧʽ)�����ɸó��������ӷ���ʽ______________����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��Ksp[Al(OH)3]=3��10 -34����
(4)��ˮAlCl3(183������)����ʪ��������������������ʵ���ҿ�������װ���Ʊ���

װ��B��ʢ�ű���NaCl��Һ,��װ�õ���Ҫ������ ��F���Լ��������� ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ _��
(5)��Mg��Cu��ɵ�1.96g�����Ͷ�����ϡ�����У���ַ�Ӧ������ȫ�ܽ�ʱ�ռ�����ԭ����NO����0.896L����״��������Ӧ�����Һ�м���2mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ���������γɳ���������Ϊ g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com