
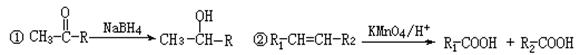

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
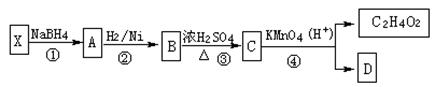
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
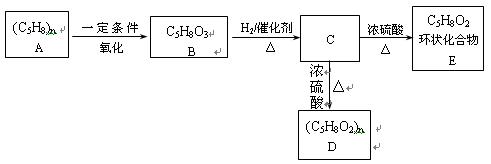
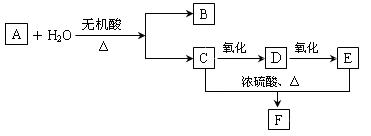
 ��������__________��
������Ϊ��__________��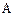 �ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________��
�ĵ�������ƣ�ϵͳ������Ϊ__________��D�Ľṹ��ʽΪ��__________��Bת��ΪC�ķ�Ӧ����Ϊ__________�� ת��Ϊ
ת��Ϊ �Ļ�ѧ����ʽ____________________��
�Ļ�ѧ����ʽ____________________��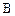 ��ͬ���칹��
��ͬ���칹��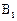 ������ֻ��һ���������ܷ���ˮ�ⷴӦ����
������ֻ��һ���������ܷ���ˮ�ⷴӦ����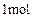
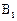 ������������Һ��Ӧ������ܵ�
������������Һ��Ӧ������ܵ�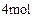
 ����
���� ���п��ܵĽṹ��ʽΪ��__________��
���п��ܵĽṹ��ʽΪ��__________���鿴�𰸺ͽ���>>
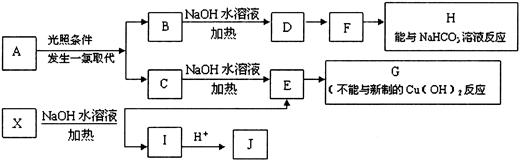
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
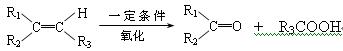
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

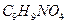
 ��һ�������¿�ˮ��Ϊ
��һ�������¿�ˮ��Ϊ ��
��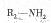 ����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
����F��ǿ��ͳ�ʱ����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 �Է�������Ϊ314�IJ����� �֡�
�Է�������Ϊ314�IJ����� �֡��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com