
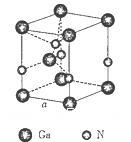
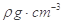 ���г����㵪���ؾ����߳�a�ı���ʽ��a=_______cm��
���г����㵪���ؾ����߳�a�ı���ʽ��a=_______cm��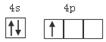
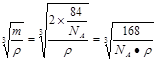
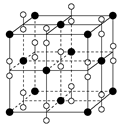
 ����2�����ڵڢ�A.�ڵڢ�A��Ԫ���У�����Nԭ�Ӱ뾶��С��ԭ�Ӻ�����������Ӵ��ڰ�������ȶ�״̬���������һ�������������Ԫ�����ڱ��ڢ�A����������Ԫ���е縺������Ԫ����ԭ�Ӱ뾶��С��BԪ�ء���3����CaCl3��NH3��һ�������·�Ӧ�Ʊ������أ����������غ㶨�ɿɵø÷�Ӧ�Ļ�ѧ����ʽΪGaCl3��NH3=GaN��3HCl�� ��4������������ʯ�������Ƶľ���ṹ�����ʯ��Cԭ��֮���Թ��ۼ���ϣ���ԭ�Ӿ��塣���Ե������е�ԭ������ԭ��֮���Թ��ۼ����ϣ�����������ԭ�Ӿ��塣��5�� ��ÿ������4��N�γɹ��ۼ������ĸ�N������������ṹ��ÿ��N��4��Ga�γɹ��ۼ������ĸ�Ga������������ṹ�����Ե���������ԭ�ӵ��ӻ���ʽΪsp3����ԭ�ӵ���λ��Ϊ4. ����ÿ�������к���Ga��8��1/8+1=2,����N��4��1/4+1=2.��ÿ�������к���2��GaN�������ؾ����߳�a����
����2�����ڵڢ�A.�ڵڢ�A��Ԫ���У�����Nԭ�Ӱ뾶��С��ԭ�Ӻ�����������Ӵ��ڰ�������ȶ�״̬���������һ�������������Ԫ�����ڱ��ڢ�A����������Ԫ���е縺������Ԫ����ԭ�Ӱ뾶��С��BԪ�ء���3����CaCl3��NH3��һ�������·�Ӧ�Ʊ������أ����������غ㶨�ɿɵø÷�Ӧ�Ļ�ѧ����ʽΪGaCl3��NH3=GaN��3HCl�� ��4������������ʯ�������Ƶľ���ṹ�����ʯ��Cԭ��֮���Թ��ۼ���ϣ���ԭ�Ӿ��塣���Ե������е�ԭ������ԭ��֮���Թ��ۼ����ϣ�����������ԭ�Ӿ��塣��5�� ��ÿ������4��N�γɹ��ۼ������ĸ�N������������ṹ��ÿ��N��4��Ga�γɹ��ۼ������ĸ�Ga������������ṹ�����Ե���������ԭ�ӵ��ӻ���ʽΪsp3����ԭ�ӵ���λ��Ϊ4. ����ÿ�������к���Ga��8��1/8+1=2,����N��4��1/4+1=2.��ÿ�������к���2��GaN�������ؾ����߳�a����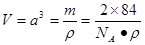 �����������ã�
�����������ã�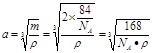 ��
��

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CCl4��SiCl4���۵� |
B�����ǻ�����ȩ��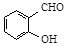 ���Ͷ��ǻ�����ȩ�� ���Ͷ��ǻ�����ȩ��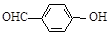 ���ķе� ���ķе� |
| C��SO2��CO2��ˮ�е��ܽ�� |
| D��H2SO3��H2SO4������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��H2O | B��CO2 | C��SO2 | D��BeCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
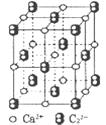
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | �� | �� | �� | �� | �� |
| ��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
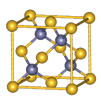
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��C3H8��̼ԭ�Ӷ����õ���sp3�ӻ� |
| B��O2��CO2��N2���ǷǼ��Է��� |
| C��ÿ��N2�У�����2���м� |
| D��CO��һ�ֵȵ�����ΪNO�������ĵ���ʽΪ[��N??O��]�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com