
���� ��1���ɦ�=$\frac{M}{{V}_{m}}$��֪��Ħ���������ܶȳ����ȣ����ܶ���������һ�룬���������ƽ��Ħ������Ϊ16g/mol�����з���ʽ�����⣻
��2������n=CV�������ʵ����ʵ������ٸ���V=nVm��������������
��3��������ƽ��ʹ�÷������������룬���̵������������̵��������������������ҩƷ����=��������+�������������λ�÷ŷ����������̵�����=���̵�����+������������е�ʽ���м��㣻
��4������n=$\frac{V}{{V}_{m}}$=$\frac{m}{M}$�������ʵ��������ÿ�����Ӻ��е�ԭ����Ŀ���㺬��ԭ�������ʵ�����
��5������SO42-Ҫ�ų�̼��������ӵĸ��ţ�������������ӿ��Ժͱ����ӷ�Ӧ�������ᱵ�������ش�
��� �⣺��1�����������ܶ���������һ�룬�ɦ�=$\frac{M}{{V}_{m}}$��֪��������ƽ��Ħ������Ϊ32g/mol��$\frac{1}{2}$=16g/mol��
�����������xmol H2��ymolCO����$\frac{2x+28y}{x+y}$=16��
�����ɵ�x��y=6��7��
������������ٷֺ���Ϊ��$\frac{6}{7+6}$��100%��46%��
�ʴ�Ϊ��46%��
��2������£�����Ħ�������22.4L/mol��V=nVm=0.6mol/L��0.5L��22.4L/mol=6.72L��
�ʴ�Ϊ��6.72��
��3�������̵�����=���̵�����+�����������֪����������=ҩƷ����+���������������ҩƷ����=��������-������������ʵ�ʳƵ�þ�۵�����=24g-0.4g=23.6g��
�ʴ�Ϊ��23.6��
��4����0.5mol�����к��е�ԭ�ӵ����ʵ���Ϊ2mol���ڱ�״����22.4L������̼�����ʵ���Ϊ1mol�����е�ԭ�ӵ����ʵ���Ϊ3mol����4��ʱ9gˮ�����ʵ���Ϊ0.5mol�����е�ԭ�ӵ����ʵ���Ϊ1.5mol�� ��0.2mol�����к��е�ԭ�ӵ����ʵ���Ϊ1.4mol����������ԭ�Ӹ�������С�����˳�������Ǣܢۢ٢ڣ�
�ʴ�Ϊ��B��
��5������SO42-Ҫ�ų�̼��������ӵĸ��ţ�����Ҫ�������������ᣬ��������������ӿ��Ժͱ����ӷ�Ӧ�������ᱵ�����������Ȼ�����Һ�����������ɫ������֤������������ӣ�
�ʴ�Ϊ�����BaCl2��Һ��
���� ���⿼�����ʵ����ļ��㡢ʵ��������ʹ�á����ӵļ��飬��Ŀ�ѶȲ�������ѧ���ķ��������ͼ��������Ŀ��飬ע�����������ܶ���Ħ�������Ĺ�ϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ�����н��п�ȼ������ȼ������ʵ��ʱ���������鴿�����ȼ | |
| B�� | ������Ũ��մ��Ƥ���ϣ�Ӧ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ | |
| C�� | ����ϡ����ʱ����������Ͳ�м�һ�������ˮ�����ڽ�������������Ũ���� | |
| D�� | �������ὦ�����У�Ӧ������ˮ��ϴ����ϴ��գ�۾� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������н�����Ӧ | B�� | ���������� | ||
| C�� | ����֧��ȼ�� | D�� | ����Ư������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
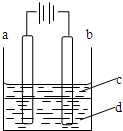 ����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռ���Һ��Ӧ�Ƶ�Fe��OH��2��ɫ������������ͼ��ʾʵ��װ�ã�����Ƶô�����Fe��OH��2��ɫ��������֪�������Ϸֱ�Ϊʯī������
����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռ���Һ��Ӧ�Ƶ�Fe��OH��2��ɫ������������ͼ��ʾʵ��װ�ã�����Ƶô�����Fe��OH��2��ɫ��������֪�������Ϸֱ�Ϊʯī�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ����Ħ����� | C�� | Ħ�� | D�� | Ħ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ô���ʩ�����Խ�����β����CO��NOת��Ϊ������ | |
| B�� | ��¯ˮ���к��е�CaSO4��������Na2CO3��Һ�������������ȥ | |
| C�� | �뵼����ҵ����һ�仰������ɳ̲���û����������оƬ�IJ����Ƕ������� | |
| D�� | ��ֹ���귢������Ҫ��ʩ֮һ��ʹ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Ӧ����ȡ����Ӧ
��Ӧ����ȡ����Ӧ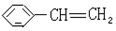 ʹ��ˮ��ɫ�ķ�Ӧ����ʽ
ʹ��ˮ��ɫ�ķ�Ӧ����ʽ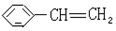 +Br2��
+Br2��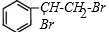 ��Ӧ���ͼӳɷ�Ӧ
��Ӧ���ͼӳɷ�Ӧ ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com