
����Ŀ��I ��һ���л���X����ṹ��ʽΪ��HO��CH2CH=CH��COOH���Իش��������⣺
(1)X�еĺ���������������_______________��_______________��
(2)��X�м�������ƣ���������Ӧ�Ļ�ѧ����ʽ��_______________��
(3)�����X�м���NaOH��Һ����������Ӧ�Ļ�ѧ����ʽ��___________��
(4)���й���X��˵������ȷ����________________��
��X�������ᷢ��������Ӧ�������봼����������Ӧ
��X�ܹ�ʹ��ˮ��ɫ��������ʹKMnO4������Һ��ɫ
��X�ܹ��������۷�Ӧ�������ܷ����Ӿ۷�Ӧ
II.����ʽΪC3H6O2���л����ж���ͬ���칹�壬�������е�����X��Y��Z��W�����ǵķ����о������������Ƿֱ��������ʵ���Լ�����ʵ���¼���£�
NaOH��Һ | ������Һ | ����Cu(OH)2 | ������ | |
X | �кͷ�Ӧ | ������ | �ܽ� | �������� |
Y | ������ | ������ | ���Ⱥ���ש��ɫ���� | �������� |
Z | ˮ�ⷴӦ | ������ | ���Ⱥ���ש��ɫ���� | ������ |
W | ˮ�ⷴӦ | ������ | ������ | ������ |
�ش��������⣺
��1��д��X�Ľṹ��ʽ________�� W��ϵͳ������________.
��2����Y��һ�������·��������ڵ���ˮ��Ӧ�Ļ�ѧ����ʽ________________
��Z��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_______________________________
���𰸡� �ǻ� �Ȼ� HO��CH2CH=CH��COOH+2Na��NaO��CH2CH=CH��COONa+H2�� HO��CH2CH=CH��COOH+NaOH��HO��CH2CH=CH��COONa+H2O �� CH3CH2COOH ������� ![]()
![]() CH2=CHCHO��H2O HCOOCH2CH3��NaOH
CH2=CHCHO��H2O HCOOCH2CH3��NaOH![]() HCOONa��CH3CH2OH
HCOONa��CH3CH2OH
��������(1)HO-CH2CH�TCH-COOH�к���������Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
(2)�ǻ����Ȼ��������Ʒ�Ӧ��������������ʽΪHO-CH2CH�TCH-COOH+2Na��NaO-CH2CH�TCH-COONa+H2�����ʴ�Ϊ��HO-CH2CH�TCH-COOH+2Na��NaO-CH2CH�TCH-COONa+H2����
(3)�ṹ��ֻ���Ȼ����������Ʒ�Ӧ������ʽΪHO-CH2CH�TCH-COOH+NaOH=HO-CH2CH�TCH-COONa+H2O���ʴ�Ϊ��HO-CH2CH�TCH-COOH+NaOH=HO-CH2CH�TCH-COONa+H2O��
(4)����ΪX�����ǻ����Ȼ�����������ᷢ��������Ӧ�������봼����������Ӧ������ȷ����X����̼̼˫�����ɷ����ӳɷ�Ӧ��������Ӧ���ʴ�����X����̼̼˫�����ɷ����Ӿ۷�Ӧ���ʴ��ʴ�Ϊ���١�
II.����ʽΪC3H6O2���л���X��Y��Z��W,���ǵķ����о�����,��X����NaOH�����кͷ�Ӧ�����ܽ�Cu(OH)2,˵������-COOH,��XΪCH3CH2COOH��Y�ܷ���������Ӧ,˵������-CHO,����Na������Ӧ��������,˵������-OH,YΪCH3CHOHCHO;Z���ܷ���ˮ��,���ܷ���������Ӧ,˵������-OOCH,Ϊ������,��ZΪHCOOCH2CH3,W��ˮ��,��������,WΪCH3COOCH3,(1)��������������֪��,XΪCH3CH2COOH��ΪW��CH3COOCH3�� W��Ϊ�����������ˣ�������ȷ����: CH3CH2COOH;���������
(2)��YΪCH3CHOHCHO ����Y��һ�������·��������ڵ���ˮ��Ӧ�Ļ�ѧ����ʽ��. ![]()
![]() CH2=CHCHO��H2O
CH2=CHCHO��H2O
����ΪZΪHCOOCH2CH3����Z��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ![]() ,��ˣ�������ȷ����:
,��ˣ�������ȷ����:![]()


 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ҩƷ������Ȼҩ����ǣ� ��
A. ��˾ƥ�� B. ��ù�� C. ����ҩ D. ��Ƽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����װ���г�����з�̪���Ȼ�����Һ��X��Y��Ϊʯī�缫����Ӧһ��ʱ�����װ��X��������Һ�ȱ�졣�����ж�����ȷ����
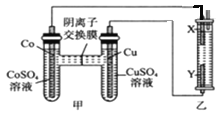
A. ��������Cu�缫��Y��X��Co�缫
B. �ܣ�Co���Ľ����Ա�ͭ��ǿ
C. ����ʱ��SO42-��Cu�缫�ƶ�
D. ת��0.2mol e-����������0.2molNaClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú��Һ�����Ժϳɼ״��������й�˵����ȷ����
�١�������:C(s)+2H2O(g)![]() CO2(g)+2H2(g) ������H1=+90.1 kJ��mol-1
CO2(g)+2H2(g) ������H1=+90.1 kJ��mol-1
�ڴ�Һ����:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ������H2=-49.0 kJ��mol-1
CH3OH(g)+H2O(g) ������H2=-49.0 kJ��mol-1
�۴�Һ����:CO2(g)+2H2(g)![]() CH3OH(g)+1/2O2(g) ��H3=a kJ��mol-1
CH3OH(g)+1/2O2(g) ��H3=a kJ��mol-1

A. ��Һ������ʹ�ô���,��Ӧ�Ļ��Ea����H2����С
B. ��ӦC(s)+H2O(g)+H2(g)![]() CH3OH(g)����H=+41.1 kJ��mol-1
CH3OH(g)����H=+41.1 kJ��mol-1
C. ��H2>��H3
D. ��ͼΪ�״�ȼ�ϵ�صĹ���ԭ��ʾ��ͼ,�����ĵ缫��ӦΪCH3OH-6e-+6OH-![]() CO2��+5H2O
CO2��+5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����Cl2��H2��CO2��NO��NH3������ѡ��һ�����壬��������ʵ���ҿ�������ͼװ����ȡ���������ռ������Ժ�ˮ����������ȡ������Ļ�ѧ��Ӧ����ʽΪ_____________��

��2����֪��
��������װ����ȡ1,2 - �������飨��ɫҺ�壬�ܶ�2.18 g��cm-3���ۡ��е�Ϊ9.79�桢131.4�棬������ˮ�����Թ�d��װ��Һ��(���渲������ˮ)��

��eװ�õ�������____________�����۲쵽_________����ʱ���Թ�d�з�Ӧ����������
�����ñ�ˮ�����ձ��е���ˮ����������IJ���ȫ�����______________��
��ʵ�������Ҫ�����Թ�d�еĴֲ�Ʒ�������Ⱥ�˳����______�����ţ�
A.���� B.ˮϴ C.�ø�������� D.10%NaOH��Һϴ E.ˮϴ
��ʵ������40%��ϩ����Ħ������ΪM����Һ50g���Ƶò�Ʒm g������ϩ���ϳ�1,2 - ��������IJ���Ϊ_____________���ú�M��m�Ĵ���ʽ��ʾ����
�����ʵ��֤������Ӧ����ƿ�еĻ����Һ����Cl-��PO43-������ʵ�鷽������֪���Ȼ���������������ɫ�������ᱵ����ɫ����KSP�ֱ�Ϊ1.77��10-10��8.88��10-17 ��3.4��10��23��
��_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��I��һ�ָ߷��ӻ������ϳ�·����ͼ��


��֪����![]()
�ڻش��������⣺
��1��A������Ϊ_______��ϵͳ����������G���еĹ�����_____��д���ƣ���
��2����Ӧ�ٵķ�Ӧ������________��
��3��I�Ľṹ��ʽΪ_____________________________��
��4����Ӧ�ڵĻ�ѧ����ʽΪ__________________________________��
��5����������������G��ͬ���칹�干��___________�֡�
����G������ͬ�����ţ� �����ڷ����廯����
��6�����������ϳ�·�ߣ����һ������ȩ���״���J(E��ͬϵ������Է���������EС14��Ϊ��Ҫԭ�ϣ����Լ���ѡ���Ʊ�![]() �ĺϳ�·��______________��
�ĺϳ�·��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ������������ģʹ�õĽ��������ǵĻ������ڿ�ѧ�о���ҵ�����о���������;����ش��������⣺
(1)��Ԫ�������ڱ��е�λ����____��ͭ�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ______ ��
(2)��ï��[Fe(C5H5)2]����ɫ�����壬���������Ե���ζ�������ԡ��۵�172.5��173�棬100�������������е�249�档�ݴ��ж϶�ï����������Ϊ______________��
(3)������CuSO4��5H2O���Ľṹ����ͼ��ʾ:

ͼ�����߱�ʾ_____________��SO42-�����幹����__________��NO3-��Nԭ�ӵ��ӻ����������___________��Oԭ�ӵļ۵����Ų�ͼΪ__________________��
(4)���Цġ��á�������ͬ�������壬��ͼ�����ǵľ���ṹͼ�����־�������ԭ����Χ�����������ԭ�Ӹ���֮��Ϊ______��

(5)ij�־��д���ܵ�ͭ�Ͻ�������������ܶѻ��Ľṹ��������Cuԭ�Ӵ������ģ�Auԭ�Ӵ��ڶ���λ�ã���ԭ�ӿɽ��뵽��Cuԭ����Auԭ�ӹ��ɵ��������϶�С�����Cuԭ����Auԭ�ӵ�ͬ�������þ��崢���ľ����ṹ�� CaF2�Ľṹ�������ṹ��ͼ�����ƣ��þ��崢���Ļ�ѧʽΪ________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��PbO�������ᣬ����ǿ����Һ���ڹ�ҵ����;�㷺������������Ǧ�ס�ɱ����ȡ�ij������Դ��ҵ�Ժ�Ǧ��������Ҫ��Pb��PbO��PbSO4�ͼ�������PbO2����ϡH2SO4Ϊԭ���Ʊ��ߴ�PbO�Ĺ�������������

��1����Ǧ�����е�PbO2��PbSO4�У�Ǧ�Ļ��ϼ۷ֱ�Ϊ____________��____________��
��2�����ܹ�����Ϊ�˼ӿ��ܽ����ʣ����˼���FeSO4�������⣬���ɲ�ȡ�Ĵ�ʩ��____________________����дһ������
��3����ҺA�к��е���Ҫ��������____________________�������ӷ�������
��4��������̷�������Ҫ��Ӧ�����ӷ���ʽΪ________________________________��
��5����ȴ�����˺�����õĹ������ϴ�Ӳ�������ʵ����ϴ��ʱ�����õ��IJ����������ձ���_________��_________����������Ƿ�ϴ�Ӹɾ��ķ�����________________________________��
��6��PbO�ܽ���NaOH��Һ�У�����ƽ����PbO(s)+NaOH(aq)![]() NaHPbO2(aq)��PbO���ܽ��������ͼ��ʾ����ϸ����ߣ������ɴ�ƷPbO�õ��ߴ�PbO�IJ�����_______________________________��
NaHPbO2(aq)��PbO���ܽ��������ͼ��ʾ����ϸ����ߣ������ɴ�ƷPbO�õ��ߴ�PbO�IJ�����_______________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д��ʵ�����б仯�Ļ�ѧ����ʽ����ָ����Ӧ���͡�
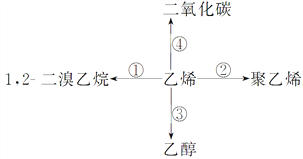
д����Ӧ�ķ���ʽ����Ӧ���͡�
��____________________________��_______________��
��____________________________��________________��
��____________________________��________________��
��____________________________��_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com