
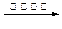 C6H12O6+6O2 b��CO2 + 3H2
C6H12O6+6O2 b��CO2 + 3H2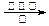 CH3OH +H2O
CH3OH +H2O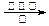 CH3COOH d��2CO2 + 6H2
CH3COOH d��2CO2 + 6H2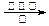 CH2==CH2 + 4H2O
CH2==CH2 + 4H2O Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
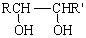
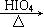 RCHO + R��CHO
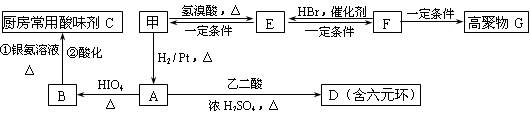
RCHO + R��CHO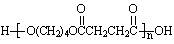 ������ƺ�����������Ϊԭ�ϣ����Լ���ѡ���ϳ�PBS���úϳ�·������ͼ��ʾ����ע����Ӧ��������
������ƺ�����������Ϊԭ�ϣ����Լ���ѡ���ϳ�PBS���úϳ�·������ͼ��ʾ����ע����Ӧ��������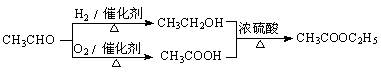
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
�鿴�𰸺ͽ���>>
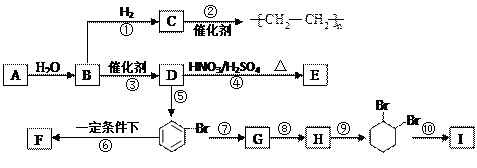
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
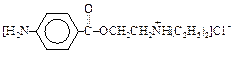
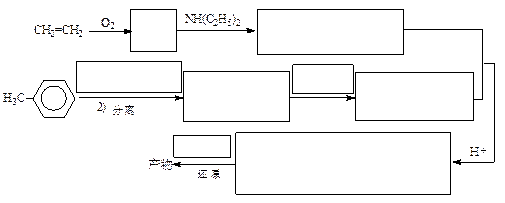
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
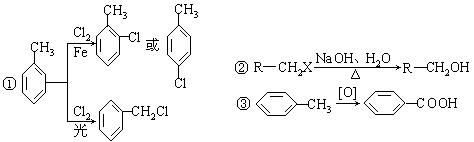
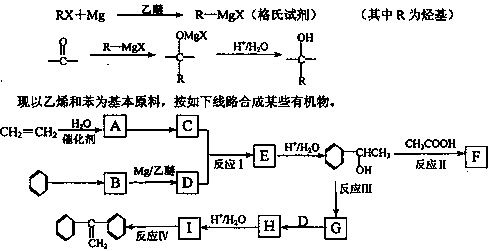
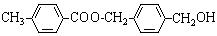 ����A�������ɷ���ͼʾ�е�һϵ�з�Ӧ������M���ڸ߷��ӻ����J��K��Ϊͬ���칹�壬N�IJ�������Ϊ����һ������ʯ�ͻ�����չˮƽ�ı�־��
����A�������ɷ���ͼʾ�е�һϵ�з�Ӧ������M���ڸ߷��ӻ����J��K��Ϊͬ���칹�壬N�IJ�������Ϊ����һ������ʯ�ͻ�����չˮƽ�ı�־��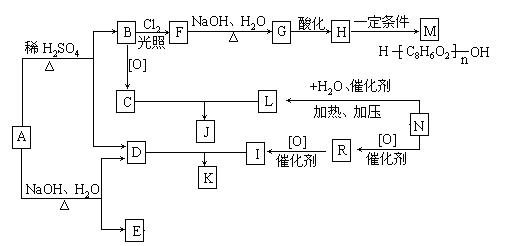
�鿴�𰸺ͽ���>>
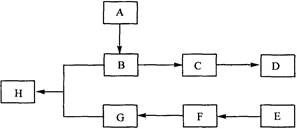
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
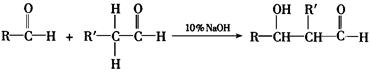
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
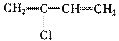 �ۺϵ�__________________________ _��
�ۺϵ�__________________________ _��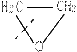 �����ߴ������ۺϵ�
�����ߴ������ۺϵ�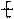 CH2CH2O
CH2CH2O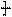 n����
n����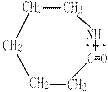 �����ۺϵ�_______________________________ ��
�����ۺϵ�_______________________________ ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com